Introduction
Particulate contamination in pharmaceutical products, e.g. drugs (both liquids and solid pills), intravenous (IV)/ infusion solutions, eye drops, and inhalers [1-7], can be a major risk for patients with serious medical conditions. In order to ensure product quality and safety, therefore, it is important to identify and eliminate particle contamination in pharmaceutical products. However, finding the source of contamination can be challenging if only visual analysis of the particles is done. Chemical analysis of the particles makes that goal easier, but the method most commonly used, scanning electron microscopy and energy dispersive spectroscopy (SEM/EDS), is complex, time-consuming, and unable to reveal particle color information [3,7]. Instead, a 2-methods-in-1 materials analysis solution, which combines optical microscopy and laser induced breakdown spectroscopy (LIBS), offers a fast, reliable, and cost-effective way to determine the shape, size, color, and composition of particles [8]. It enables simultaneous visual and chemical analysis of particle contaminants, without the need for further time-consuming sample preparation or sample transfer between several instruments. The DM6 M LIBS 2-in-1 material analysis system currently used for non-regulated environments, such as R&D and engineering labs, shows great potential in the future for root cause analysis in the pharmaceutical industry [9].
Particulate contamination in the pharmaceutical industry
Particulate contamination in pharmaceutical products, such as drugs, both liquids and solid pills/tablets, intravenous (IV)/infusion solutions, eye drops, and inhalers, can come from many different sources (undissolved residuals in solutions, packaging, seals, etc.) [1-4]. These contaminant particles can be composed of metal, glass, and synthetic (rubber) materials [1,3,4]. Such particles can be a risk to patients as they may cause sepsis, systemic inflammatory response syndrome (SIRS), organ dysfunction or failure, phlebitis, and granulomatous pulmonary arteritis [4-6].
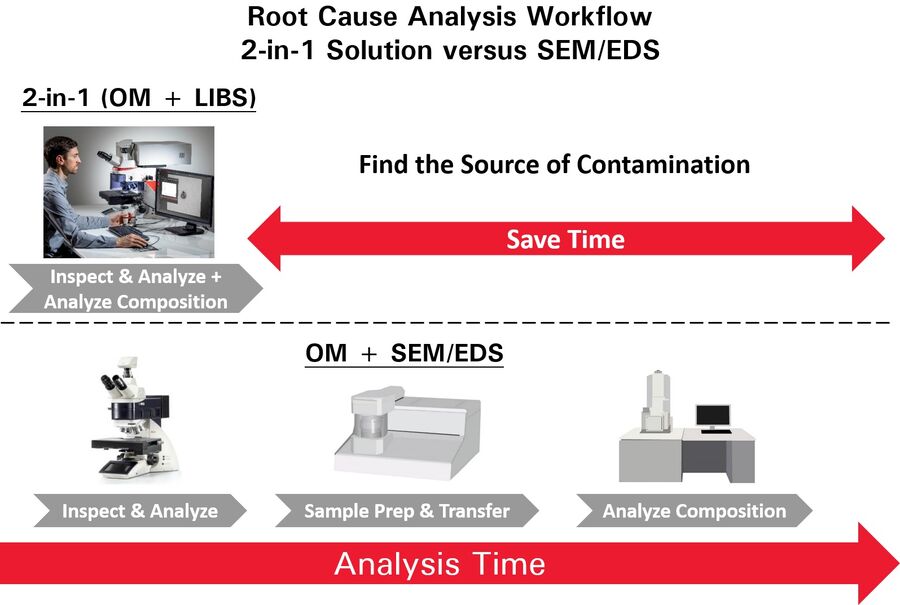
Identification of the particulate contamination is done in several steps. The first step is visual analysis performed with an optical microscope to determine the size, shape, microstructure, and color of the particles, as well as count them. The next step is chemical analysis of the particles in order to determine composition and make it easier to find out where they come from (root cause analysis). Typically, SEM/EDS is used for particle chemical analysis, however, it is expensive and time-consuming as it requires the sample to be transferred into a vacuum chamber and the particles of interest relocated. A 2-methods-in-1 solution makes visual and chemical analysis of particles more efficient (refer to figure 1).
Particle analysis
During visual analysis, the very similar appearance of different metal particles (high alloy or low alloy steel, aluminum alloys, etc.) can sometimes make it difficult to determine the source of the particle contamination. In this case, an instantaneous compositional analysis of the particles would greatly help in finding the particle source more efficiently to minimize the contamination in pharmaceutical products. However, as mentioned above, compositional analysis with methods like SEM/EDS can be slow and cumbersome.
A 2-methods-in-1 solution, like the DM6 M LIBS from Leica Microsystems, offers advantages over SEM/EDS (figure 1). For example, the true color of particles can be seen and the source of contamination can be quickly and efficiently determined using LIBS elemental analysis. When visual inspection alone is not sufficient, users can differentiate particles based on spectral fingerprints and characteristic signals found in the LIBS spectra. Therefore, LIBS can provide useful elemental information of particulate contamination related to pharmaceutical products.
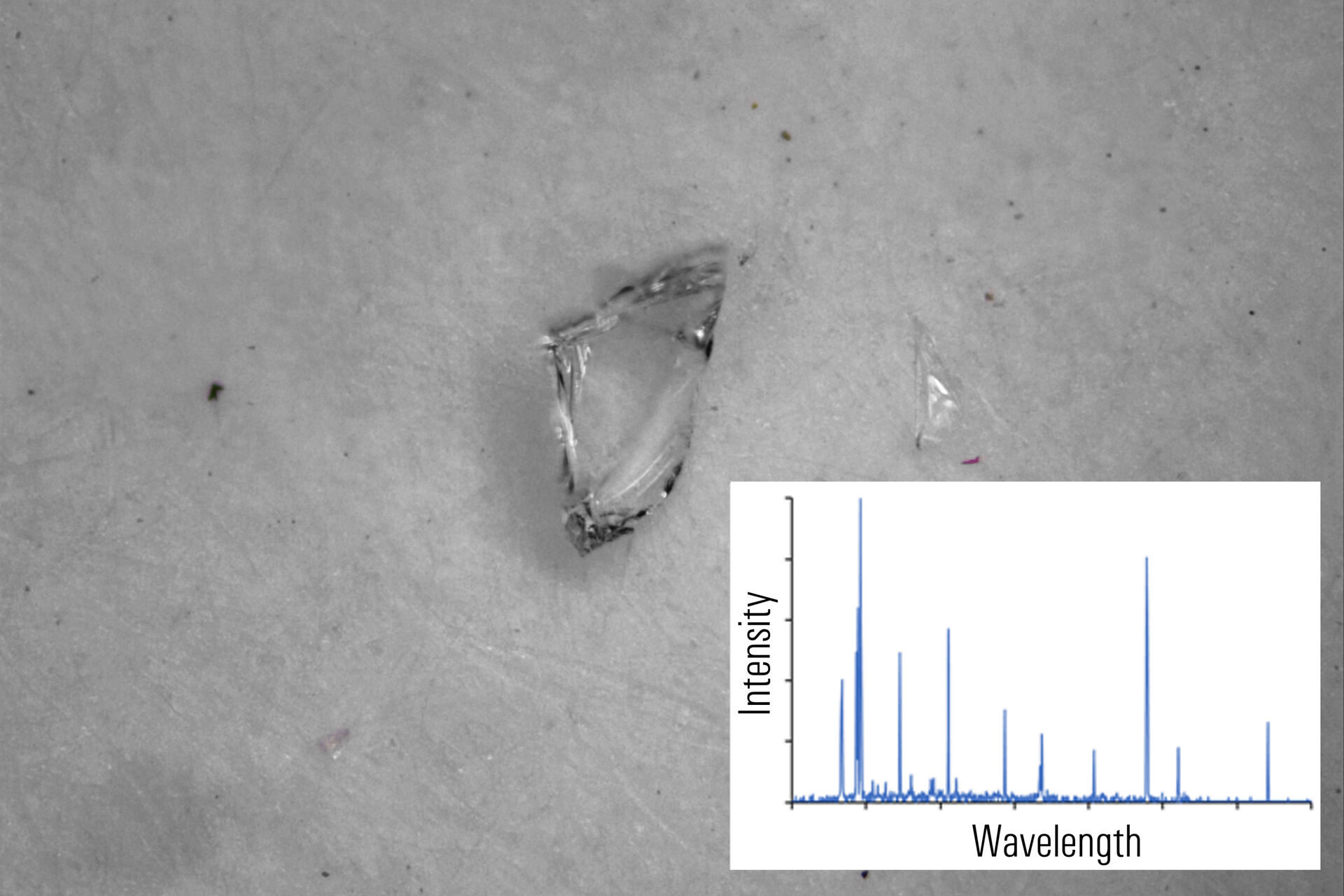
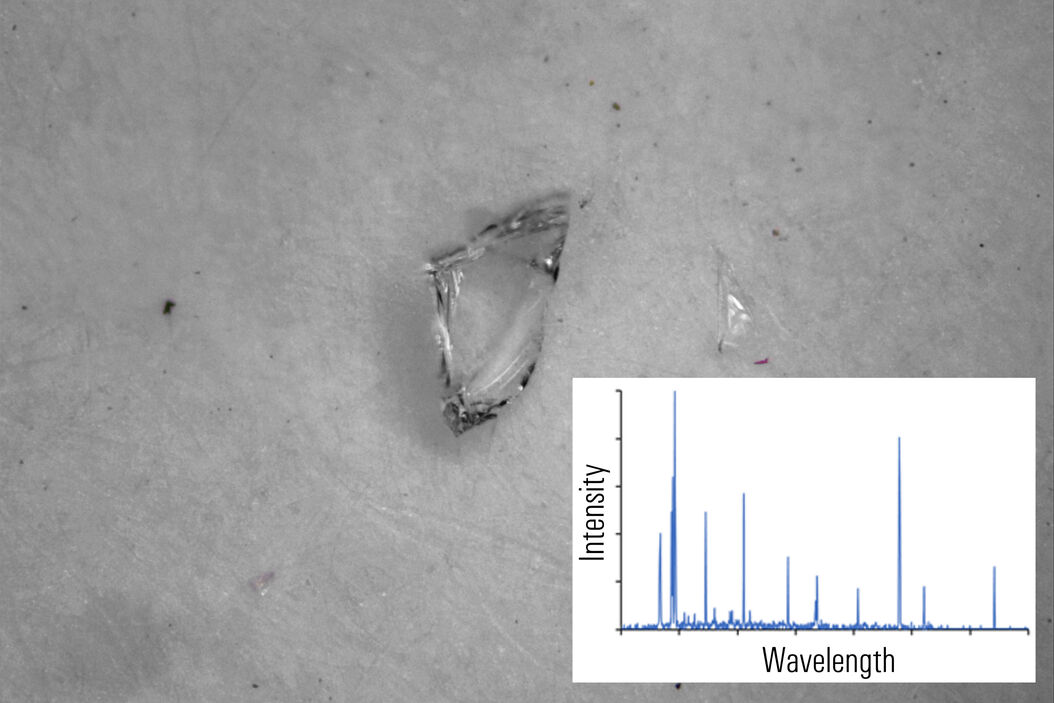
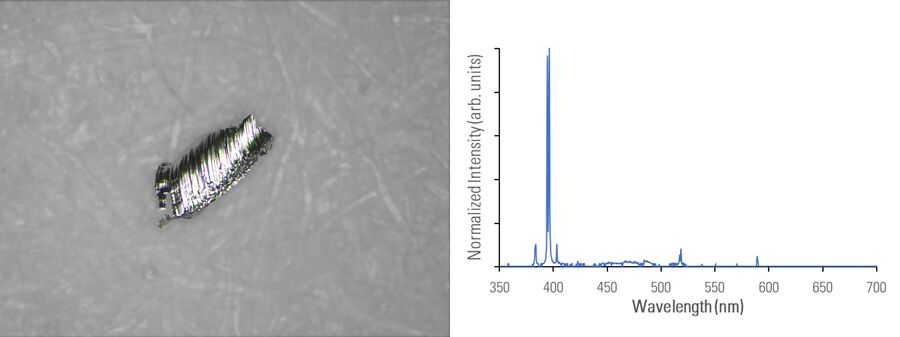
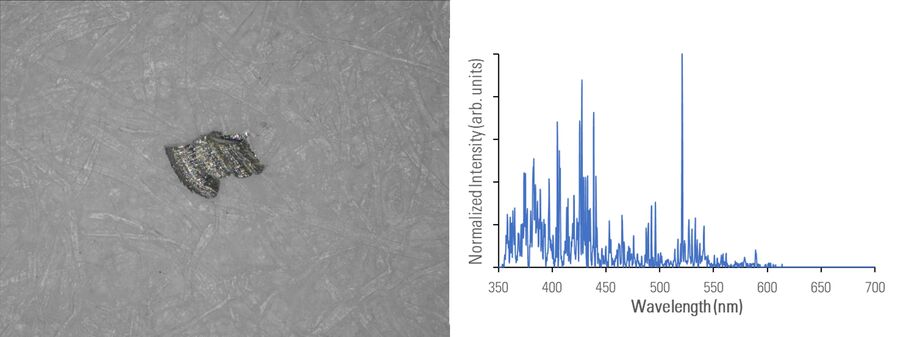
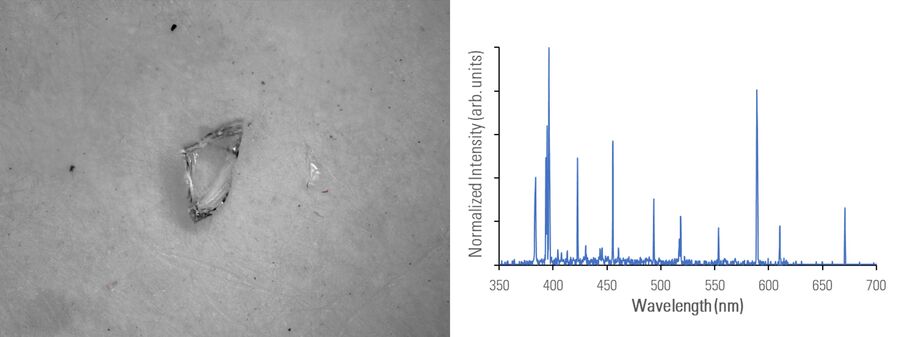
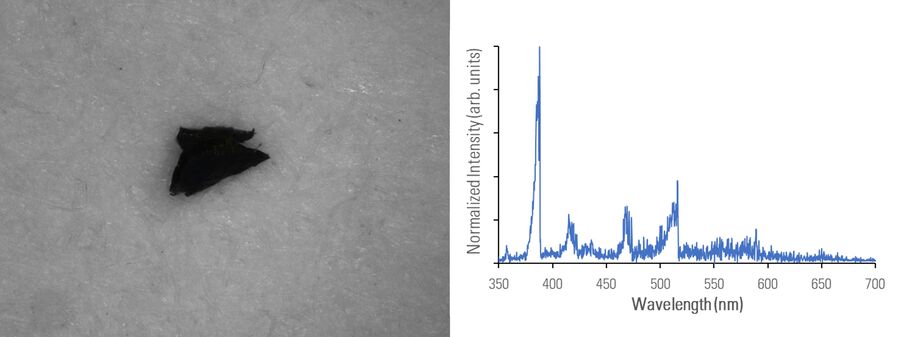
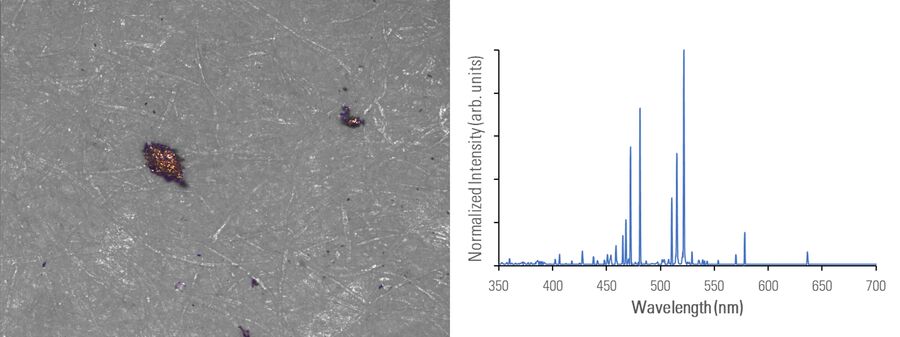
In figures 2A-E below, particles composed of different materials are imaged with optical microscopy and analyzed with LIBS using the DM6 M LIBS 2-in-1 solution.
Different metal particles (steel versus aluminum alloys) can look similar, so a rapid compositional analysis, possible with a 2-in-1 solution, would greatly help in finding the particle source more efficiently for root cause analysis in the pharmaceutical industry. Even when there is a difference in appearance, such as color (refer to figure 2D with the dark rubber particle or 2E with the gold/yellow brass particle), the assumed particle composition can still be verified with LIBS.
Summary and conclusions
A 2-methods-in-1 solution using high-resolution optical microscopy and laser induced breakdown spectroscopy (LIBS), e.g. the DM6 M LIBS, enables simultaneous visual and chemical analysis of particulate contamination. For non-regulated environments, it is a more practical solution for efficient identification and elimination of sources of contamination. A 2-in-1 solution helps save time, because it does not require additional time-consuming sample preparation or sample transfer between multiple analytical instruments. Finally, it may be possible for root cause analysis to be done more rapidly and easily with the goal of minimizing the risk to patients who use pharmaceutical products.