Introduction
The three aquatic model organisms mentioned above, zebrafish, medaka, and Xenopus, are often used in molecular and developmental biology. An adult zebrafish is shown below in figure 1.
In molecular and developmental biology, these aquatic vertebrate model organisms are widely applied to study molecular processes of development and as disease models. To study these molecular mechanisms, proteins of interest are fluorescently labeled and observed in the developing organism at the cellular or sub-cellular level over the course of hours or days.
All three model organisms described here can be easily bred and maintained in a laboratory, have short life cycles, and are amenable for genetic modifications [4-6]. Examples of these modifications are the deletion of a gene (knock-out) or the introduction of a gene (knock-in). If an exogenous gene is introduced into the genome, the result is a so-called transgenic organism. Below, we will focus on this method.
In addition, zebrafish have a specific trait that also make them useful for developmental neuroscience: the larvae of zebrafish are semi-transparent so the activity of multiple neurons can be measured simultaneously during development [7].
Key considerations for contemporary model organism experimentation
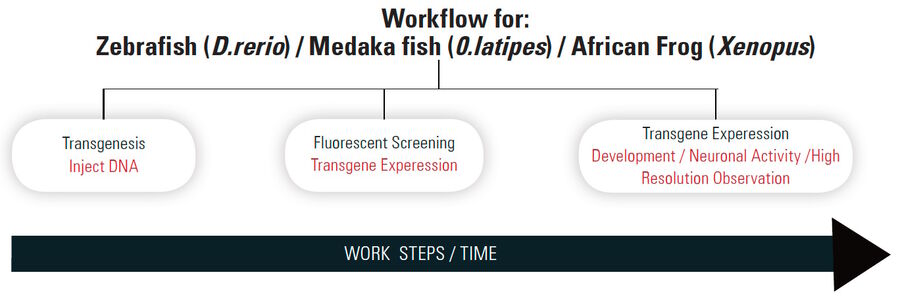
There are three common steps when doing routine work with aquaticmodel organisms, such as zebrafish:
- transgenesis
- fluorescent screening
- functional imaging.
A more detailed description of each work step is given in the section below. Efficient and reliable microscopy is needed for each of these. This sequence of steps will be referred to as "workflow" in this report.
Most countries have well defined regulations for animal safety when used for scientific experiments. Switzerland has such regulations as well 8]. To adhere to these regulations, it is advantageous to have efficient and fast screening of transgenic embryos and rapidprocessing of the adult zebrafish which generated the embryos.
As individual adult zebrafish cannot be permanently labeled, at least not at the present time, males and females that are cross-bred, to assess their embryos while screening for transgenics, need to be kept in individual holding tanks until their embryos are well characterized. The faster the embryos’ traits can be determined:
- the sooner the adults can be put back into proper housing tanks
- the number of individual tanks in the facility, and the amount of work for the staff, can be minimized
- and only zebrafish with the desirable traits would be maintained, avoiding the need to keep unnecessarily high numbers of fish for experimental work.
Faster, accurate characterization of the zebrafish embryos leads to a more efficient, cost-effective way to maintain these model organisms.
There are two key factors for fast and accurate embryo characterization:
- efficient fluorescence detection of sometimes dimly glowing transgenes
- and an efficient, convenient way of imaging the embryos for screening.
In practical terms, fluorescence microscopes that detect weak florescence signals and make them visible to the eye in an uncomplicated way are the optimal tools to achieve this goal.
Work steps
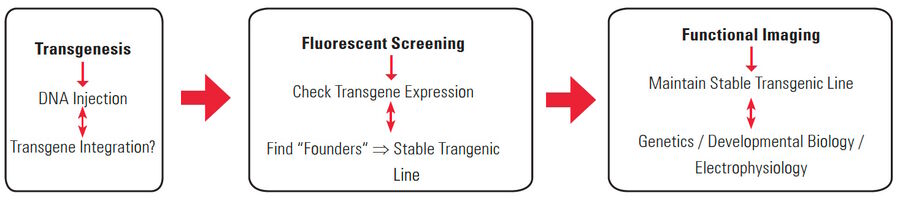
Transgenesis
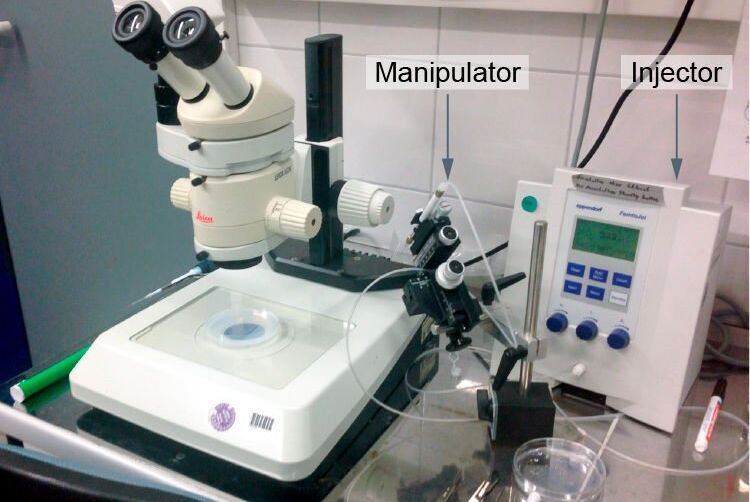
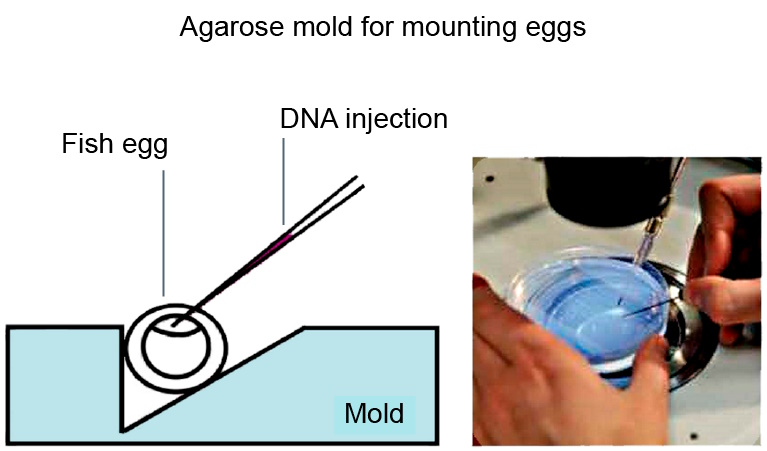
Genetic modifications in zebrafish, medaka, and xenopus are typically carried out by microinjection of DNA, RNA, or dyes (as for plasmids, mRNAs, morpholinos, siRNAs, etc.) [9]. These manipulations are efficiently supported by the optical magnifications achievable with a stereo microscope, such as the M50, M60, or M80 [10]. If DNA is injected into a cell and incorporates into the genome (transgene), this results in a transgenic animal.
Fluorescent screening
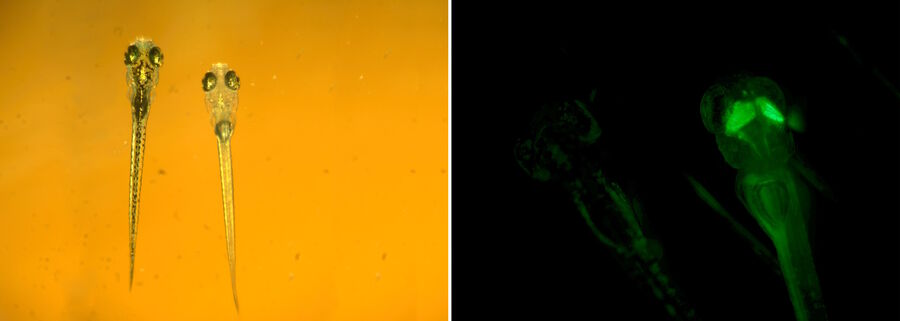
As the organism develops into the larval stage, successful integration (into the genome) and expression of the transgene is evaluated. A part of the transgene is usually a gene for a fluorescent protein, such as green, red, or yellow fluorescent protein [11]. Therefore, screening of potentially transgenic larvae is commonly done with a fluorescence stereo microscope, such as the MZ10 F [12], M165 FC, or M205 FA [13].
Functional imaging
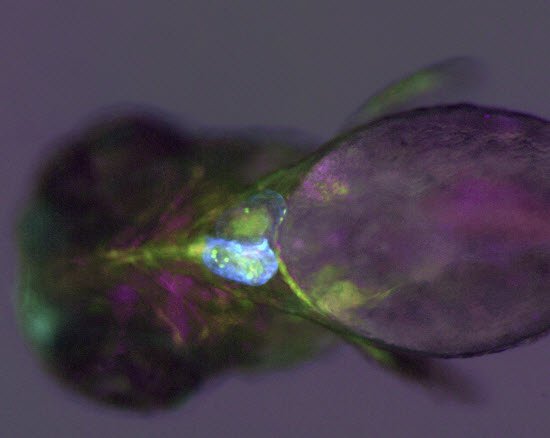
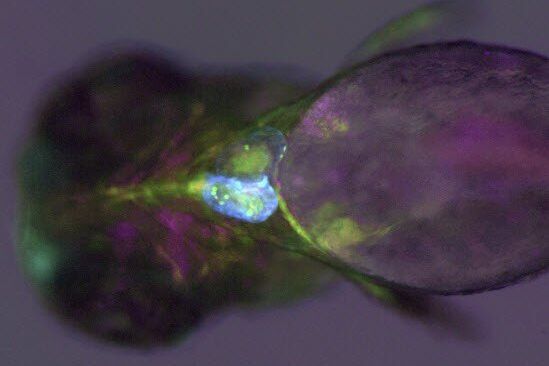
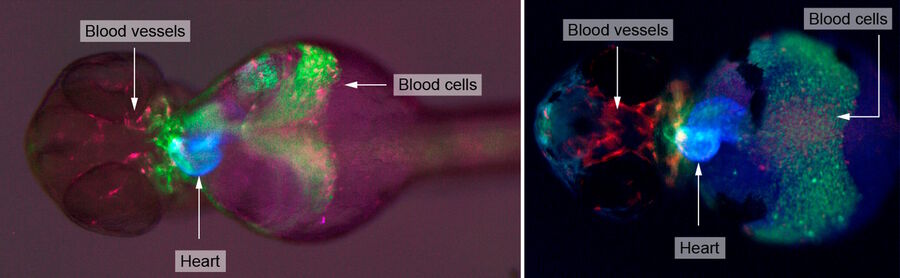
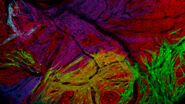
An example of functional imaging is electrophysiological investigation via Ca2+ signaling in various types of cells. Injection of an organism with synthetic Ca2+ indicators [14], frequently using a micromanipulator, enables studies of neuronal activity in neurons and glial cells. Calcium indicators can also be genetically expressed and imaged in intact or semi-intact organisms due to the semi-transparent nature of developing zebrafish larvae. These experiments are frequently done using multiphoton fluorescence microscopy [15].
During development, the organism is often imaged with a stereo microscope and, in some cases, manipulation and preparation for further experiments is performed with it as well. For high-resolution observation of transgenic, XFP-expressing (three or more fluorescent proteins simultaneously) [16] organisms or immunostained preparations, macroscopes or confocal, multiphoton, and lightsheet microscopes, such as the STELLARIS series [17], are commonly used.
Photos of a zebrafish laboratory (Mosimann Lab, IMLS) showing several stereo microscopes.
Key considerations for optimizing workflow efficiency with aquatic model organisms
Transgenesis
When generating transgenics, it is important to use a transmitted light base to visualize the internal structure of the eggs. As many eggs have to be injected in order to obtain a few "founders" where the transgene was successfully incorporated into the germ line, the injections normally take several hours, which makes a relaxed working posture very important. During the injection step, it is important to arrange the eggs under the microscope so that the operator has a good overview, allowing him/her to inject in a fast and efficient way. Finally, a large microscope base enables researchers to move around several dishes with less risk of them falling over the edge. Typically micromanipulators, such as the Eppendorf InjectMan NI 2, or Narishige MMO-220A are used. Commonly used injectors are the ASI MPPI-3, Eppendorf FemtoJet, and Parker Picospritzer, to name a few examples.
Routine manual stereo microscopes used for transgenesis
Increasing workflow efficiency with Leica routine stereo microscopes
- Larger field of view (FOV) / object field (OF) (the viewing area) → eyepieces with a field number (FN) of 23 available that give a 20% increase or more in FOV, compared to those with a FN of 21 or smaller;
- Less focusing required while viewing a specimen/sample → large depth of field at low magnification;
- Max resolution of 1.6 μm (numerical aperture (NA) of 0.21) with the M80 and max resolution of 2.2 μm (NA 0.15) with the M60 and M50;
- High quality images with achromatic or plan achromatic objectives [19];
- Compact design → small footprint allows the microscope to fit into a limited space;
- Increased comfort and productivity: less muscle strain when using a focus drive with adjustable torque → depending on the overall weight of the microscope system, the torque of the focus knobs can be adjusted to users’ preferences;
- Avoid fatigue with Ergo modules → enable users to maintain better posture while working;
- Clean and compact overall setup of the instrument → cables integrated into the focus column for the camera, illumination, and motorized focus.
Increasing workflow efficiency with the TL3000 transmitted light base
- Versatile contrast methods: brightfield and one-sided darkfield illumination;
- Move multiple specimens/samples around easily and more space for hands around the objective when doing manipulation and sorting and during dissection → large flat surface for specimen placement;
- Simple to operate, ideal for routine applications.
Increasing workflow efficiency with the TL5000 Ergo transmitted light base
- Versatile contrast methods: very homogeneous brightfield, optimized Rottermann Contrast and a low-reflection darkfield;
- Easier handling, work faster and save time – automatic aperture adjusts itself automatically to the zoom optics to achieve optimal contrast;
- Study entire organisms with high precision – large field of view (FOV) with as much as 65 mm diameter;
- Work fatigue-free on a specimen and handle manipulators more easily – extremely flat, ergonomic LED light base;
- Reproducibility due to full encoding using Leica Application Suite (LAS) and LAS X software [20];
- Bright, homogeneous and color-neutral illumination independent of intensity – made possible with the latest LED technology.
Fluorescent screening
Although the bioengineering of fluorescent proteins has produced several enhanced GFP variants, it is possible that the desired transgene is expressed at low levels. This low-level expression can be due to both biological processes and technical issues. For these reasons, fluorescence detection sensitivity can make the difference between finding and missing a transgenic organism correctly expressing the transgene. In addition, as mentioned above, it is important for efficient, accurate zebrafish embryo trait characterization to have efficient fluorescence detection of weak transgene signals.
During screening and characterization of developing zebrafish, it is often necessary to compare organisms with the same lighting and microscope optics settings. Thus it is essential to store these settings for easy, efficient recall and to ensure reproducibility. Understanding the fluorescence patterns of transgenic zebrafish, which can be quite abstract when seen in isolation, often requires switching between fluorescence and the transmitted lighting of the base. Programming of the microscope controller, such as the Leica SmartTouch or foot pedal, and working with an encoded microscope with transmitted light base, such as the Leica M165 FC or Leica M205 FA with the Leica TL5000 Ergo, simplifies this task. It also enables rapid assessment of cell position and embryo orientation after fluorescence imaging.
Autofluorescence
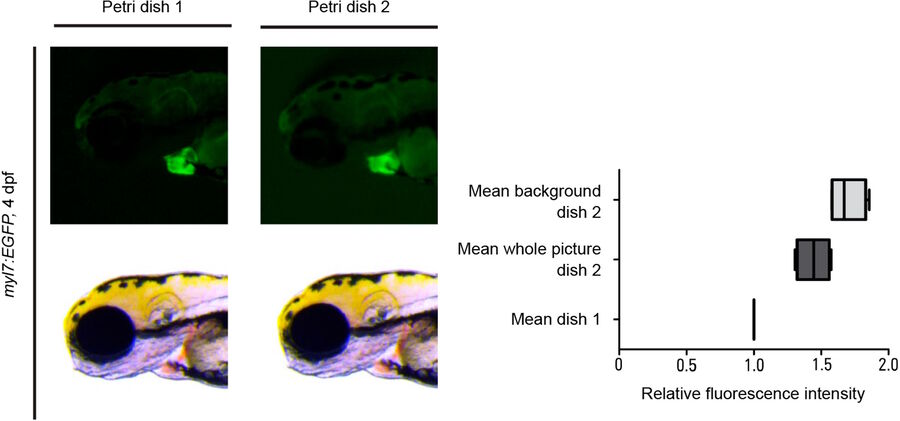
When observing very weak fluorescence signals during experiments, it is important to eliminate or minimize as much as possible background autofluorescence coming from the material of the container in which the animals are imaged, normally a petri dish. After an extensive search involving multiple commercial suppliers, the Mosimann laboratory found plastic petri dishes with minimal autofluorescence, sufficiently rigid plastic, well-closing lids, and an advantageous price. The combination of these petri dishes with a special procedure for prepping them to eliminate contamination leads to minimal background autofluorescence during experimental observation. Further details about these petri dishes can be obtained from the Mosimann laboratory.
Routine manual stereo microscopes used for fluorescence screening
A Leica stereo microscope with the fluorescence module (EL6000 [21] external fluorescence source) or the MZ10 F [12] fluorescence stereo microscope with TL3000 [18] transmitted light base:
Increasing workflow efficiency with the MZ10 F
- Excellent resolving power (max 1.33 μm) with high numerical aperture (max NA = 0.25) and 10:1 zoom range;
- Intense fluorescence illumination and highest fluorescence signal-to-noise (S/N) ratio with TripleBeam technology;
- High quality images with plan achromatic and plan apochromatic objectives [19];
- Rapid 4-position filter changing system (FLUOIII);
- Wide range of standard and custom filters for nearly any fluorescence technique;
- User protection against UV radiation exposure;
- Wide variety of objectives and accessories available
Research fluorescence stereo microscopes used for fluorescence
Increasing workflow efficiency with the M165 FC and M205 FA
- Go from overview to finest detail with a zoom optics magnification range of 16.5:1 or 20.5:1;
- Highest fluorescence signal to noise (S/N) ratio with TripleBeam technology;
- Resolution down to 1.10 μm (max NA 0.3) with the M165 FC and 0.96 μm (max NA 0.w35) with the M205 FA;
- Achieve the highest resolution and depth of field currently possible for a 3D image viewed with a stereo microscope → FusionOptics [22] available with the M205 FA;
- High quality images with plan achromatic or plan apochromatic objectives [19];
- Reproducible results obtained easily due to instrument encoding;
- Distortion free observation of immersed or embedded specimens with the PLAN APO 2.0x CORR objective which allows elimination of refractive index mismatch;
- Complete comfort and ease-of-use when doing complex experiments with the fully automated M205 FA;
- Rapid 4-position filter changing system (FLUOIII);
- Wide range of standard and custom filters for nearly any fluorescence technique;
- Wide variety of objectives and accessories available.
Functional imaging
Functional imaging often involves electrophysiological investigations and studies of neuronal activity, experimentally exploiting Ca2+ signaling, or dissection of the organism. Due to the semi-transparent nature of zebrafish larvae, these type of experiments are normally done using multiphoton fluorescence microscopy [15] or confocal microscopy [17]. For dissection, imaging is often done with stereo microscopy. Some examples of functional imaging are shown below.
Summary and conclusions
Zebrafish (Danio rerio) [1], medaka fish (Oryzias latipes) [2], and african clawed frog (Xenopus laevis) [3] are aquatic model organisms commonly used for developmental biology research [4-6].
The routine workflow for zebrafish, medaka fish, or african clawed frog involves multiple steps using stereo microscopy:
- transgenesis, injecting DNA into the eggs of the organisms to generate "founders";
- fluorescent screening, observing the larvae for correct transgene expression to find “founders”
- functional imaging, characterizing the stable transgenic line with lower or higher resolution microscopy, or studying the electrophysiology and neuronal activity.
Transgenesis is usually done with stereo microscopy and the characterization of transgenic lines with fluorescent stereo microscopy. For higher resolution imaging to obtain subcellular details, electrophysiology, or neuronal activity, confocal or compound microscopy is used.
This report refers to examples of scientists and technicians working with aquatic model organisms and shows different possible setups with a large range of microscopes and accessories. Because the demands of each laboratory can vary widely, a large range of configurations and instruments are available to address specific tasks in the workflow or even enable more work steps to be performed by one instrument. This short report presents recommended workflows based upon the experiences of different labs and can be a very useful reference or guideline when setting up or expanding a developmental biology lab using zebrafish, medaka fish, or little African clawed frog.
References
- Danio rerio, information on fishbase.org.
- Oryzias latipes, information on fishbase.org.
- Xenopus laevis, information on amphibiaweb.org. www.amphibiaweb.org
- J.M. Spitsbergen, M.L. Kent, The State of the Art of the Zebrafish Model for Toxicology and Toxicologic Pathology Research - Advantages and Current Limitations, Toxicologic Pathology (2003) vol. 31, iss. 1, suppl., pp. 62-87, DOI: 10.1080/01926230390174959.
- M. Tanaka, M. Kinoshita, D. Kobayashi, Y. Nagahama, Establishment of medaka (Oryzias latipes) transgenic lines with the expression of green fluorescent protein fluorescence exclusively in germ cells: A useful model to monitor germ cells in a live vertebrate, PNAS (2001) vol. 98, no. 5, pp. 2544-2549, DOI: 10.1073/pnas.041315498.
- J.B. Wallingford, K.J. Liu, Y. Zheng, Xenopus Quick Guide, Current Biology (2010) vol. 20, no. 6, pp. R263-R264, DOI: 10.1016/j.cub.2010.01.012.
- L.E. Kuil, N.J.M. Kakiailatu, J.D. Windster, E. Bindels, J.T.M. Zink, G. van der Zee, R.M.W. Hofstra, I.T. Shepherd, V. Melotte, M.M. Alves, Unbiased characterization of the larval zebrafish enteric nervous system at a single cell transcriptomic level, iScience (2023) vol. 26, iss. 7, 107070, DOI: 10.1016/j.isci.2023.107070.
- Animal experimentation, Swiss Federal Food Safety and Veterinary Office (FSVO), blv.admin.ch.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts, P. Walter, Ch. 4 DNA, Chromosomes, and Genomes & Ch. 6 How Cells Read the Genome: From DNA to Protein, in Molecular Biology of the Cell, 5th Ed. (W.W. Norton, 2007, New York) DOI: 10.1201/9780203833445.
- Brochure, M50, M60, M80 routine stereo microscopes.
- Greb, C. Introduction to Fluorescent Proteins: An overview of their spectral and photometric characteristics, Science Lab (2023) Leica Microsystems.
- Brochure, MZ10 F modular stereo microscope.
- Brochure, Leica M165 FC, M205 FA fluorescent stereo microscopes.
- R.A. de Melo Reis, H. Rezende Freitas, F. Garcia de Mello, Cell Calcium Imaging as a Reliable Method to Study Neuron - Glial Circuits, Front. Neurosci. Sec. Brain Imaging Methods (2020) vol. 14, DOI: 10.3389/fnins.2020.569361.
- Mülter, A. Principles of Multiphoton Microscopy for Deep Tissue Imaging, Science Lab (2019) Leica Microsystems. https://www.leica-microsystems.com/science-lab/life-science/principles-of-multiphoton-microscopy-for-deep-tissue-imaging/
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW, Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system, Nature (2007) vol. 450, pp. 56–62, DOI: 10.1038/nature06293.
- Brochure, STELLARIS confocal platform.
- Product Page, TL3000 and TL5000 Ergo transmitted light bases.
- Product Page, Objective Classes.
- Brochure, Leica LAS X software
- Product Page, EL 6000 external light source for fluorescence excitation
- Goeggel, D., Schué, A., Kiper, D., Müller, C. What is the FusionOptics Technology? Leica stereo microscopes with FusionOptics combine high resolution and depth of field for ideal 3D perception of samples, Science Lab (2023) Leica Microsystems.