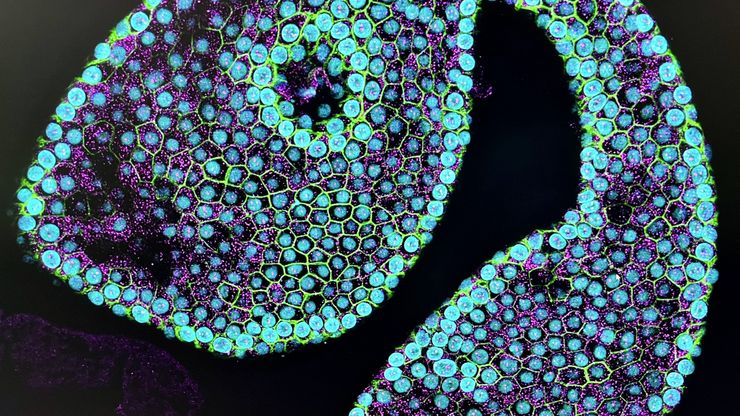
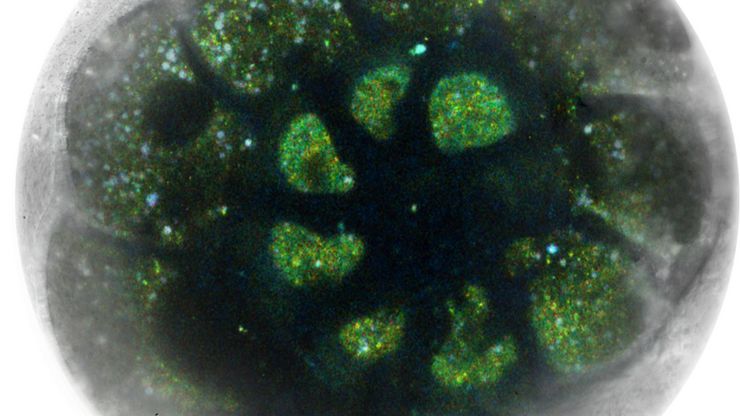
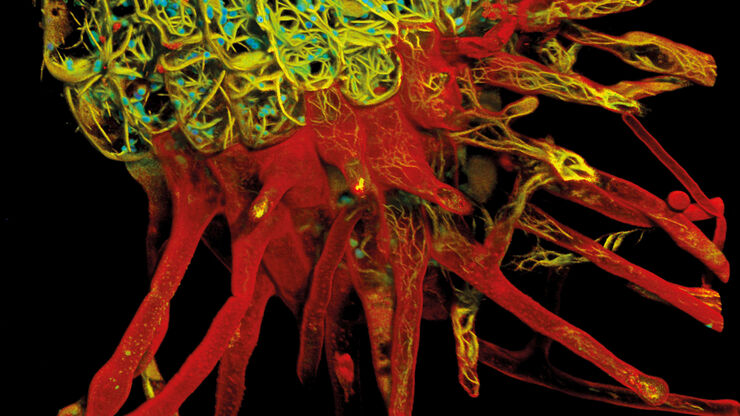
Leica and the EMBL Imaging Centre – Enabling Open Access
Throughout its history, Leica has enthusiastically developed relationships with academic and scientific research institutions to advance scientific understanding through microscopy. Now, thanks to our special partnership with the European Molecular Biology Laboratory (EMBL) in Heidelberg, researchers can gain access to cutting edge sample-prep and imaging technology.
By supporting EMBL’s drive to better understand the molecular basis of life through the provision of the latest technologies and expert support for a wide range of scientific and experimental services, Leica helps scientists push the boundaries of their research further and unlock greater insights.
Leica is among four industrial partners which helped to realize the vision of the EMBL Imaging Centre, which officially opened in 2022.
Visit the EMBL IC website to see the instruments available to research applicants.
Latest Updates
Interviews
Interview with Yassin Harim, PhD Student at German Cancer Research Center, Heidelberg
Gain insights into 3D- whole mouse brain imaging using multicolor immunofluorescence! According to our guest user from the German Cancer Research Center, using THUNDER Imager Live cell at the EMBL IC was “…the perfect solution to get very high-quality images and also to spend little time on imaging because it's just so fast to acquire each individual slide”.
Interview with Virginia Pierini – Service Manager EMBL IC, Heidelberg. Virginia Pierini is supporting the operation of the EMBL Imaging Centre regarding all its services, with a special focus on users. She is the IC’s point of contact for all users, providing help around access procedures, the project execution as well as user training.
Interview with Virginia Pierini, Service Manager EMBL IC, Heidelberg
Virginia Pierini is supporting the operation of the EMBL Imaging Centre regarding all its services, with a special focus on users. She is the IC’s point of contact for all users, providing help around access procedures, the project execution as well as user training.
Interview with Giorgia Susin, PhD Student, University of Trento, Italy
Giorgia Susin studies non-coding RNA transport in Xenopus laevis neurons using the STELLARIS microscope to unveil RNA-organelles interactions and enhance understanding of RNA transport. By performing live super-resolution imaging with TauSTED XTend for minimal light exposure, she preserved sample integrity while achieving high-resolution insights.
The EMBL Imaging Centre houses the latest state-of-the-art instrumentation from Leica and others, as well as those developed in EMBL research groups.
The EMBL IC offers researchers access to scientific experts from both academia as well as industry, providing its users with the opportunity to perform cutting-edge science with a suite of tools and support that are unavailable to most scientists.
Leica experts are on-site permanently at the EMBL IC to empower researchers to use the data from its advanced imaging systems in order to achieve groundbreaking insights.
Product Specifications
Find detailed product information for the configurations displayed at the EMBL Imaging Centre
STELLARIS 8 STED Falcon and STELLARIS STED
- Five spectrally tunable Power HyD sensitive photon counting detectors (2 HyD S, 2 HyD X, 1 HyD R)
- Confocal: tunable White Light Lasers (WLL) 440-790 nm, 405 nm STED depletion: 592 nm, 660 nm, 775 nm
- 8kHz Resonant Scanner
- Total system dead-time: 1.5 ns
- TauSTED: Tunable resolution based on lifetime (depending on sample and fluorophore: <30 nm (lateral) and <100 nm (axial). Automatic lifetime-based background suppression algorithm. Light dose reduction (WLL excitation) for all STED lines (592, 660, 775 nm). Available for 2D and 3D STED in live and in fixed specimens, also for multicolor applications. Automated workflow integrated in the LAS X software.
- The STED WHITE glycerol and water objective lenses with motCORR technology provide adaptive optical correction for aberrations introduced by sample inhomogeneities and refractive-index mismatch. The STED WHITE objective lenses provide a working distance of 300 µm:
- HC PL APO 86x/1.20 W motCORR STED WHITE
- HC PL APO 93x/1.30 GLYC motCORR STED WHITE
- HC PL APO 100x/1.40 OIL STED WHITE"
STELLARIS 8 DIVE Falcon
- Laser lines: IR: tunable 680 – 1080 nm and 680 – 1300 nm, fixed 1040 nm; Confocal: White Light Lasers (WLL) 440 – 790 nm, 405 nm, and 488 nm
- Four NDD channels equipped with Power HyD X (tunable from 380 – 800 nm), five spectrally tunable internal counting detectors (3 HyD S, 1 HyD X, and 1 HyD R)
- Upright confocal fixed-stage (DM6 CFS) stand furnished with Scientifica scanning stage
STELLARIS Cryo
- STELLARIS Scanhead with 5 HyD detectors (2 HyD S, 2 HyD X, 1 HyD R)
- Laser supply unit with super-continuum laser (440 – 790 nm) and a diode laser line with 405 nm.
- K5 sCMOS camera
- Upright DM6 confocal fixed stage microscope
- HC PL APO 50x/0,90 CRYO CLEM cryo objective lens
- 6 position motorized nosepiece for room temperature work with different objectives 10x – 100x
- Märzhäuser stage equipped with Cryo stage or sample holder for RT imaging and multiple travel ranges for cryo and room temperature.
25 l dewar with cryo pump and cryo control unit
STELLARIS Cryo is part of the Coral Cryo CLEM workflow consisting additionally of EM GP2, EM VCM plus EM VCT500 Cryo transfer shuttle or EM ACE600 coater plus EM VCT 500 cryo transfer shuttle.
MICA Microhub
- Integrated modulation contrast (IMC) and brightfield transmitted light imaging in RGB or gray scale mode
- Incident fluorescence illumination: LED 365 nm, 470 nm, 555 nm, 625 nm
- 4 simultaneous widefield detection channels with FluoSync spectral unmixing
- Confocal illumination: Laser diode 405 nm, 488 nm, 561 nm, 638 nm
- 4 simultaneous confocal detection channels (HyD FS) with FluoSync spectral unmixing
- Environmental control: Temperature (room temperature +3 °C to 45 °C), CO2 (0 – 10 %), humidity
- Closed loop water dispenser for objective immersion. Water immersion for one objective is feedback controlled and does not require any user interaction
- THUNDER Methods: Instant Computational Clearing (ICC), Small Volume Computational Clearing (SVCC), Large Volume Computational Clearing (LVCC),
- LIGHTNING Methods Basic, LIGHTNING Expert
THUNDER Imager Live Cell
- Based on the fully motorised, high-end, inverted research microscope DMi8
- High-speed positioning with the Quantum stage and Synapse real-time controller
- High-speed illumination with a multi-line LED light source
- Fast switching external filter wheel
- Adaptive Focus Control (AFC) with closed loop focus
Climate chamber ensures optimal physiological conditions for living cells
THUNDER Imager Live Cell is part of the Coral Life live cell CLEM workflow consisting of the THUNDER Imager Live Cell, the EM ICE high pressure freezer and the EM AFS2 for automatic freeze substitution.
THUNDER Imager 3D Tissue
- Based on a fully automated upright research microscope for the acquisition of multi-color 3D images
- sCMOS camera system
- Software creates blur-free large overviews of the entire tissue specimen
- Precise motorised z-focus drive to capture images in the z-direction and visualise them with the 3D Viewer
DMi8 S with TIRF module
- Fully motorised DMi8 inverted research microscope with integrated real-time controller to operate the system with microsecond precision
- Adaptive Focus Control (AFC)
- Equipped with a high-end sCMOS camera
LMD7
- Wavelength: 349 nm
- Pulse frequency: 10–5,000 Hz
- Pulse length: <4 ns
- Average pulse energy: 120 μJ
- Range of dedicated LMD objectives: 2.5x, 5x, 10x, 20x, 40x, 63x, and 150x
EM GP2
- Working temperature adjustable between +4°C and +60°C
Relative humidity adjustable to 99%
The EM GP2 is part of the Coral Cryo CLEM workflow consisting of EM GP2, STELLARIS 5 Cryo and EM VCM plus EM VCT500 Cryo transfer shuttle or EM ACE600 coater plus EM VCT 500 cryo transfer shuttle.
EM ICE
- Electrical and light stimulation module
Automatic draining of the LN2 Dewar
The EM ICE is part of the Coral Life live cell CLEM workflow consisting of the THUNDER Imager Live Cell, the EM ICE high pressure freezer and the EM AFS2 for automatic freeze substitution.
EM ACE600
- Automated, receipt-based sputter coating process
- High-quality carbon films by using carbon thread coating, carbon rod coating, or e-beam evaporation
Precise, robust, and amorphous films with sub-nanometer thickness
The ACE600 is part of the Coral Cryo CLEM workflow consisting of EM GP2, STELLARIS 5 Cryo and EM VCM plus EM VCT500 Cryo transfer shuttle or EM ACE600 coater plus EM VCT 500 cryo transfer shuttle.
EM AFS2
EM FSP (freeze substitution processor), its automatic reagent handling system
The EM AFS2 is part of the Coral Life live cell CLEM workflow consisting additionally of the THUNDER Imager Live Cell, and the EM ICE high pressure freezer.
Bring your research project to EMBL and get support by the Leica Microsystems application specialists.
EMBL related articles
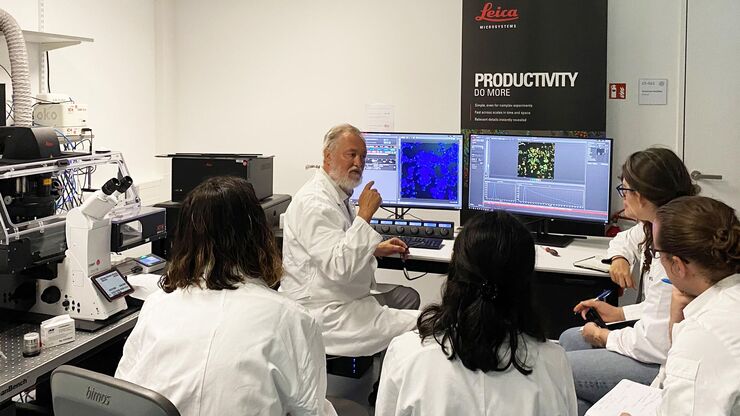
Meet the Leica Team at the EMBL IC

Robert Kirmse
Robert received his PhD from the DKFZ and University of Heidelberg. As post-doc, he worked on tumor cell invasion at BioQuant, Heidelberg and in cryo EM at the University of Colorado, Boulder. He joined Leica in 2019 as senior manager for sample preparation and site lead in Vienna. Since October 2022 he leads the Leica EMBL IC team for Scientific Innovation.

Falk Schlaudraff
Falk Schlaudraff received his PhD from the University of Ulm and subsequently worked at Qiagen. He joined Leica Microsystems in 2011, initially as Product Manager for Laser Microdissection. Over the years, he took on responsibility for additional product lines and moved into leadership roles in product, application, and workflow management. Among other things, he oversaw innovations in Laser Microdissection (LMD) and played a key role in advancing Deep Visual Proteomics (DVP). Since January 2026, he has been leading the global Application Management Life Science Research team, focusing on advanced confocal, light sheet, and widefield microscopy.

Andreia Pinto
Andreia worked as an electron microscopy specialist in Lisbon for 11 years. In 2019, she moved to London to finish her PhD and work in the fields of AI and Covid-19. Currently, she is an Advanced Workflow Specialist at Leica Microsystems and is based at the EMBL Imaging Centre in Heidelberg.