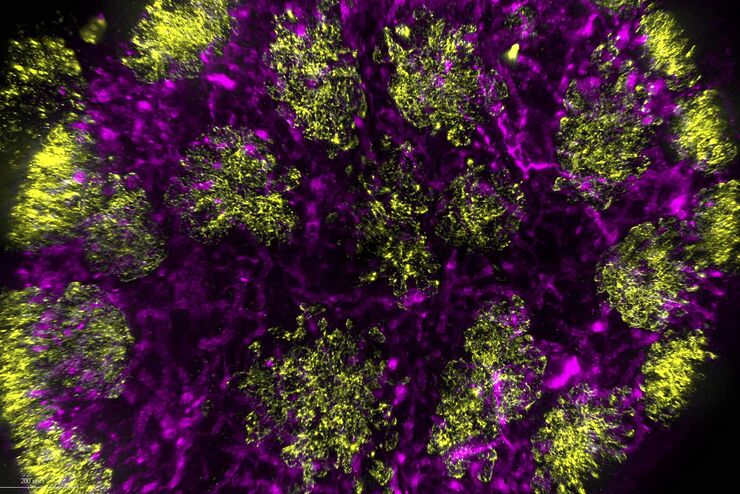
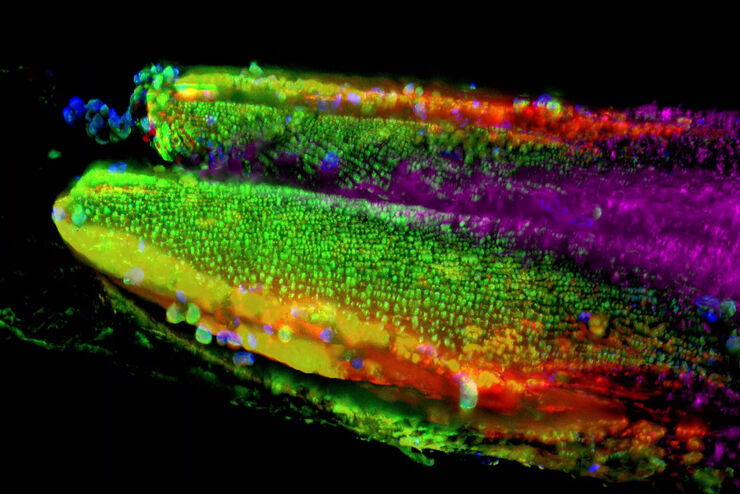
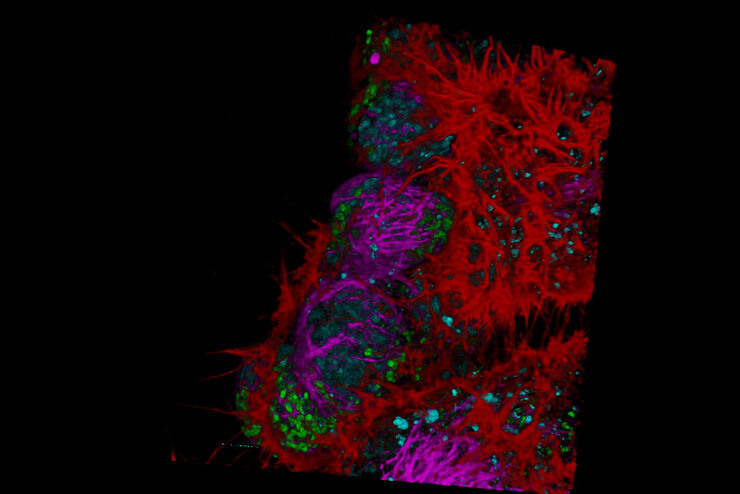
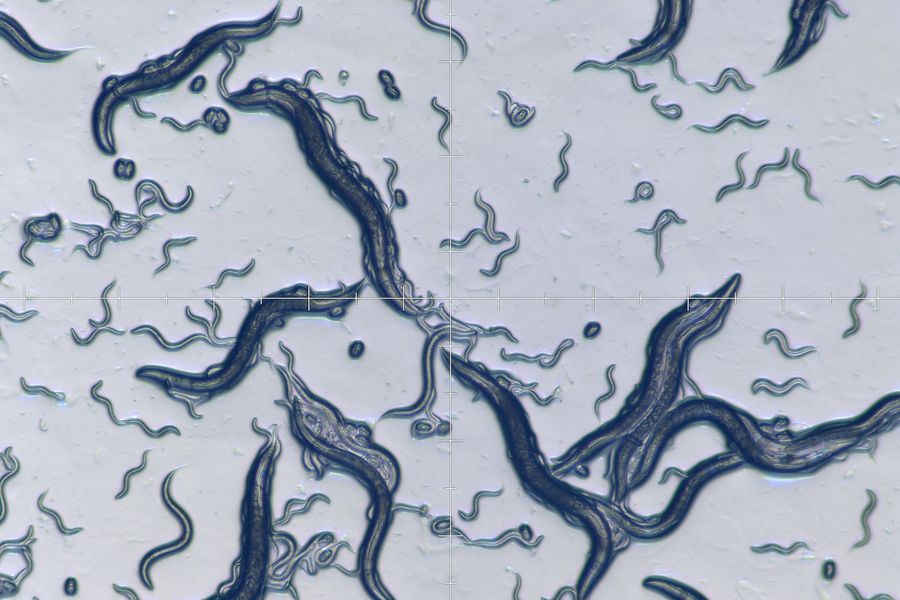
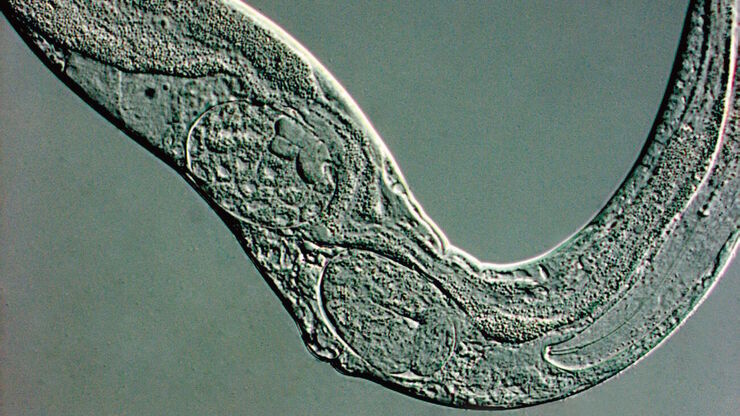
Microscopy and C. elegans research
Microscopes are essential tools while working with nematodes. They are utilized during these steps:
- “Worm picking” where worms are selected using a metal pick from agar plates to be transferred to a fresh plate for culturing or for experimental purposes
- Transgenesis (genetic modification) by microinjection of nucleic acids into worms
- Fluorescence screening where transgenic worms expressing transfected fluorescent proteins are identified
- Imaging and documentation of the worms (all stages, from embryos to adults) during experiments.
These tasks are performed using stereo, compound, or confocal microscopy.
C. elegans research Overview articles
Studying Caenorhabditis elegans (C. elegans)
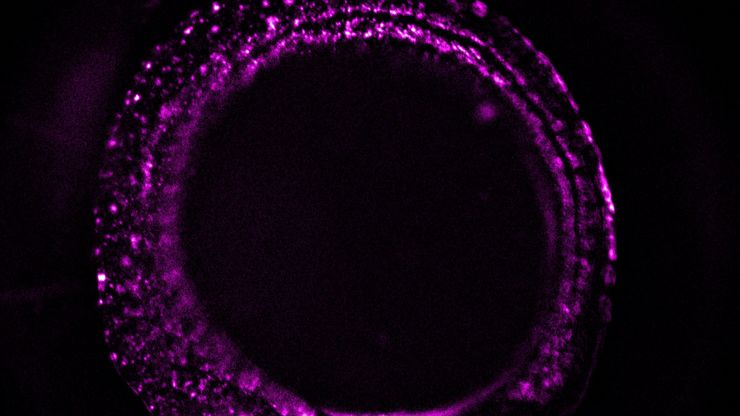
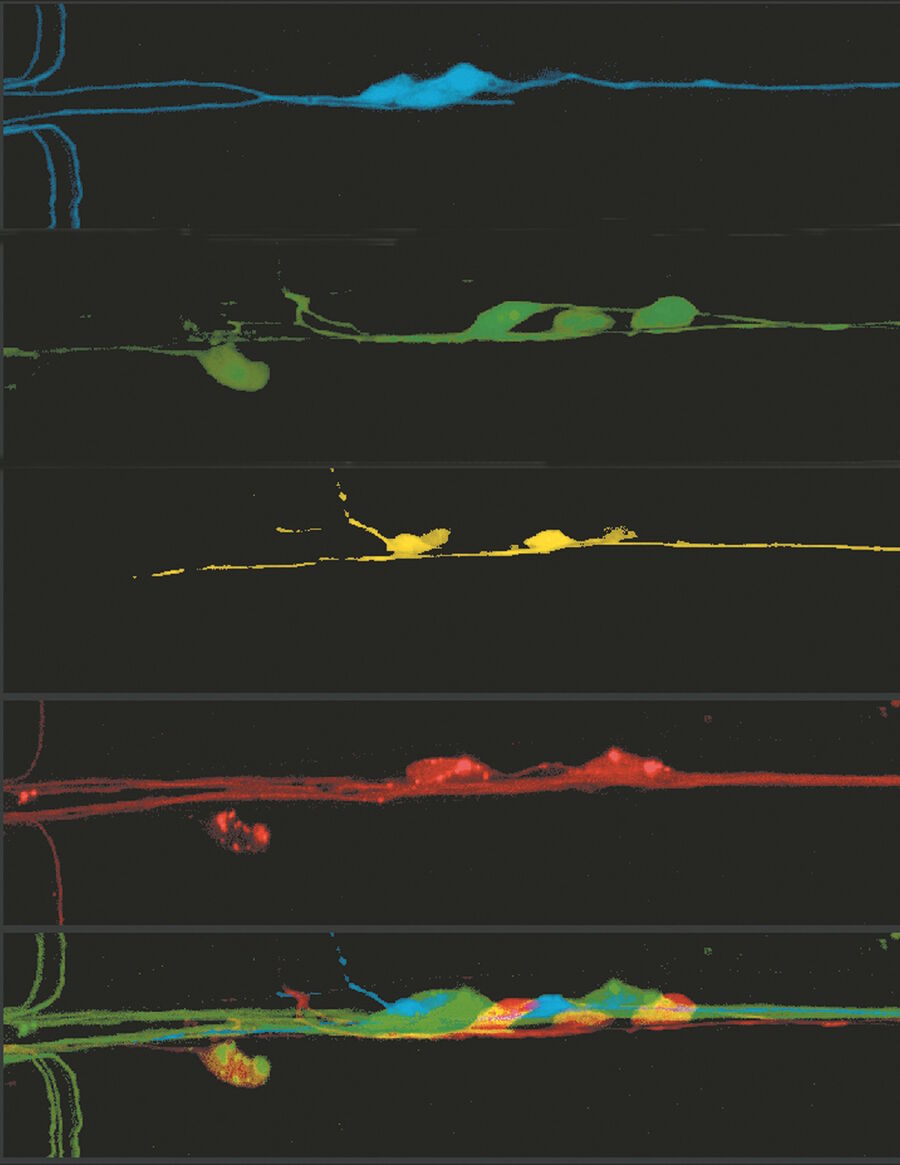
Image Gallery: THUNDER Imager
Developmental Biology Image Gallery
Fluorescence Lifetime-based Imaging Gallery
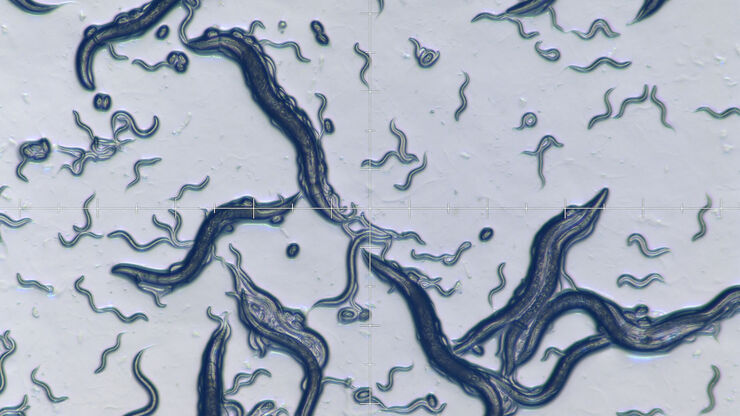
Worm picking
To “pick” an individual C. elegans with the appropriate phenotype or transgenesis or move worms from one plate to another, researchers use a stereo microscope and a small tool with a metal tip at the end.
The challenges associated with worm picking are:
- Difficulty identifying key anatomical details of worms when contrast is not good enough
- User fatigue when long hours are needed to pick worms
- Not enough space under the microscope objective when handling worms with tools
- Trouble to find worms when field of view is not large enough.
However, these challenges can be overcome with Leica microscopes which offer the following advantages:
- High optical contrast can be achieved with a transmitted light base equipped with various contrast methods, allowing observation of worm details
- Comfortable setup for users, leading to less strain and fatigue, thanks to ergonomic modules
- Sufficient space under the objective with a working distance of up to 122 mm
- Larger fields of view, making it easier to find worms.
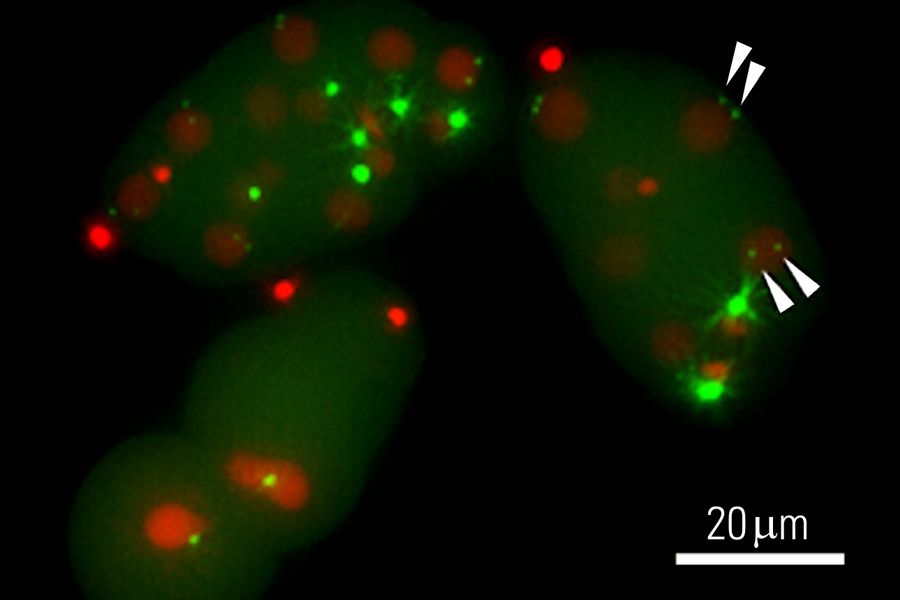
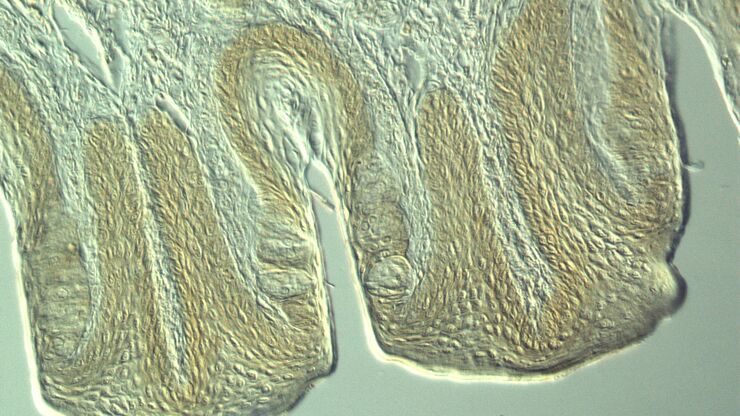
Transgenesis by microinjection
Transgenesis of C. elegans is often done by microinjection of nucleic acids. Microinjection is often performed with an inverted compound microscope outfitted with an injection setup.
There are several challenges concerning microinjection of C. elegans:
- Insufficient contrast to observe the fine anatomical details of worms which is needed for appropriate targeting
- Not having enough space to work with microinjectors
- Too much vibration when the setup is not stable.
To overcome these challenges, users can take advantage of a Leica microscope which offers:
- Optical contrast methods like DIC (differential interference contrast) or IMC (integrated modulation contrast) to clearly see where to inject the worm
- A setup which is stable against vibration (anti-vibration setup)
- The ability to use precise manipulators (Narishige or Eppendorf) for microinjection.
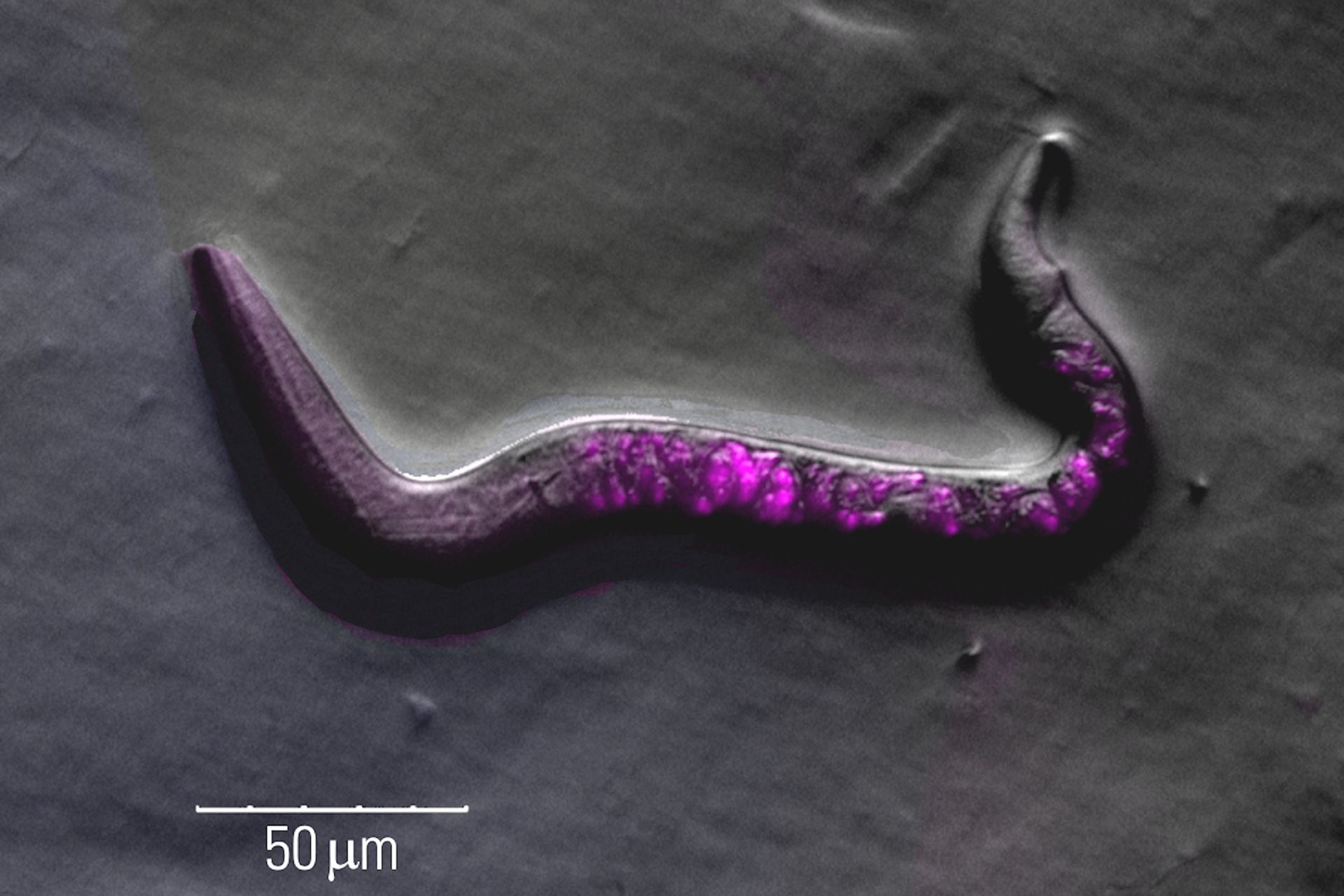
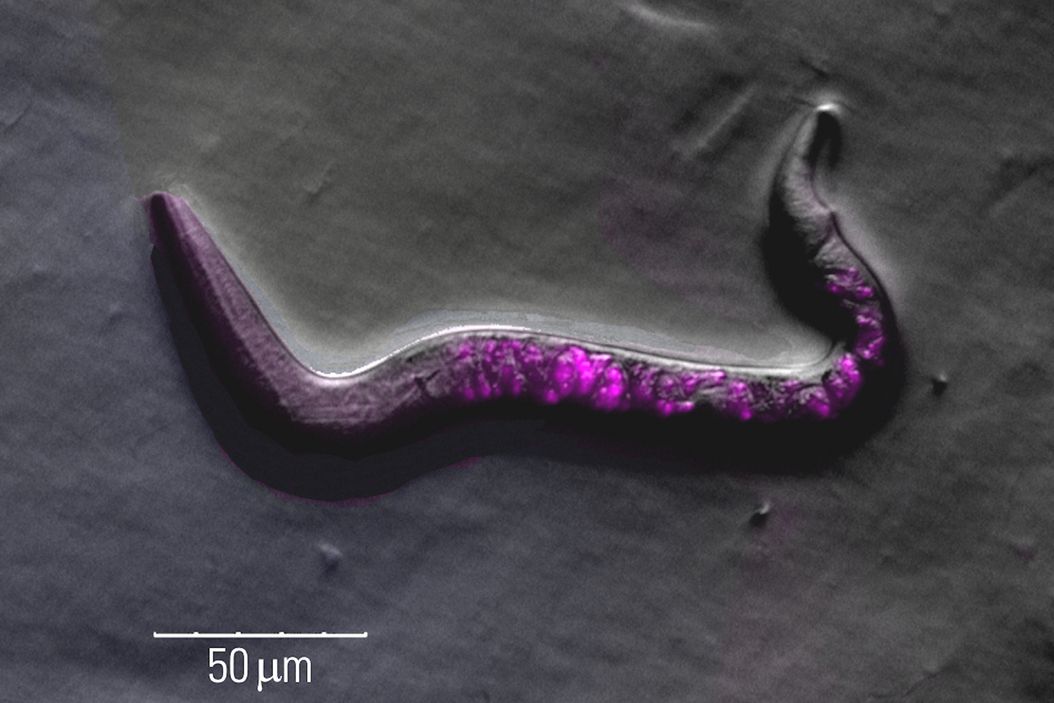
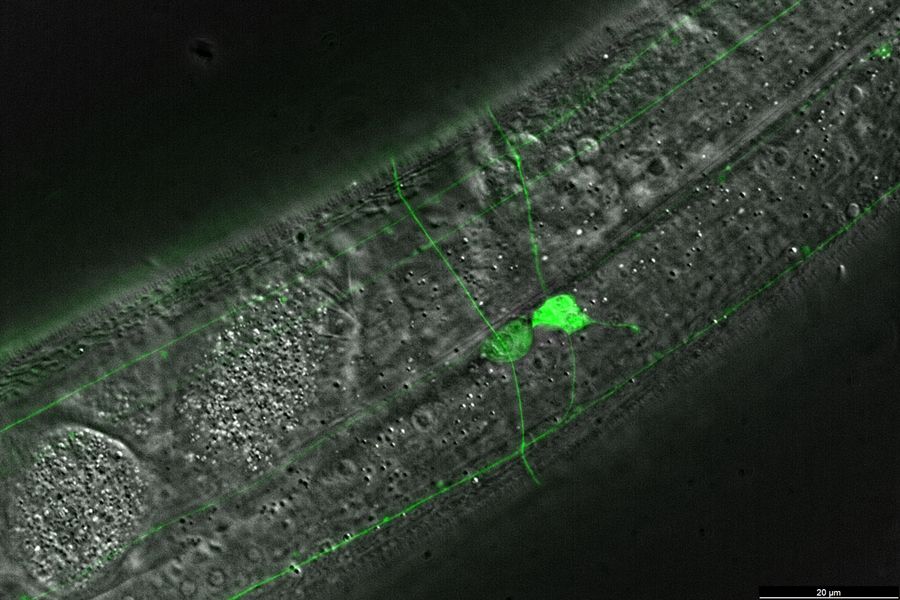
Fluorescence screening
Finding C. elegans showing successful transgenesis requires fluorescence screening with a stereo microscope.
There are certain challenges associated with fluorescence screening of worms:
- Difficulty visualizing fluorescent transgenes due to dim fluorescence signals
- A strong autofluorescence from older worms and the agar
- Problems finding worms with a small field of view leading to longer screening times.
These screening challenges can be overcome with a Leica microscope in the following ways:
- Increased signal-to-noise ratio due to a separate excitation channel with the Triple Beam technology for stereo microscopes
- Prevention of autofluorescence using a specially designed mirror available with Leica transmitted light bases
- Large fields of view and multiple contrast methods provided by Leica transmitted light bases.
Contrast methods in microscopy
Optical Contrast Methods
Phase Contrast and Microscopy
Differential Interference Contrast (DIC) Microscopy
The Polarization Microscopy Principle
Integrated Modulation Contrast (IMC)
A Guide to Darkfield Microscopes
Improving Zebrafish-Embryo Screening with Fast, High-Contrast Imaging
Epi-Illumination Fluorescence and Reflection-Contrast Microscopy
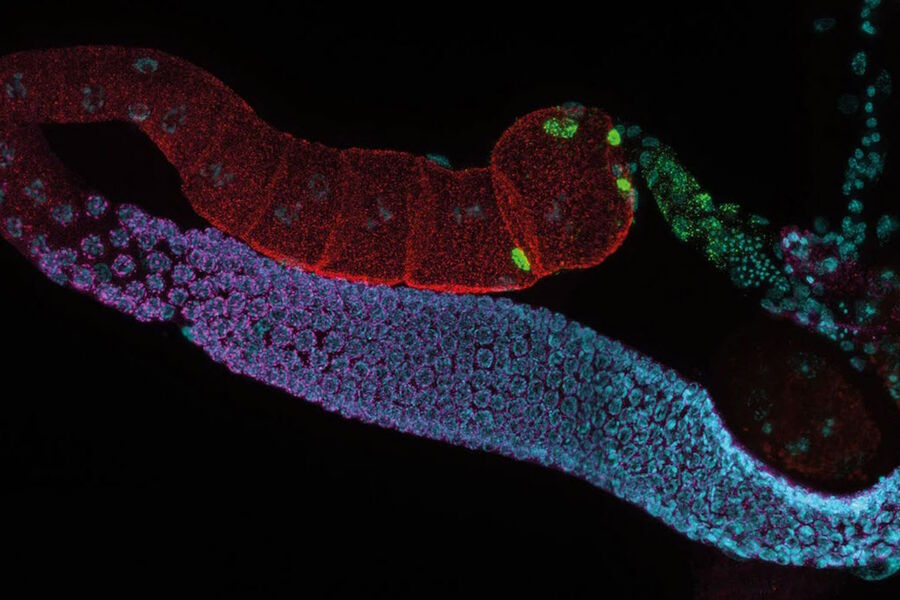
Imaging and documentation
Following the screening and identification of transgenic C. elegans specimens, high-resolution imaging and precise documentation are essential for experimental data analysis. Achieving this aim requires a microscope equipped with a high-quality camera or detector and compatible imaging software.
The main challenges concerning imaging and documentation of worms are:
- Out-of-focus blur when imaging structures deeper in worms due to light scattering
- Avoiding damage to worms from light and heat
- Visualizing dim fluorescence in live worms (including time-lapse imaging) using genetically-encoded reporters.
These challenges can be overcome with a Leica microscope. Here are the advantages:
- Visualize details deep within worms by taking advantage of Computational Clearing
- Reduce light exposure and decrease harm to worms using high quality optical elements, appropriate illumination sources, and sensitive cameras/detectors
- Facilitate live imaging by taking advantage of a good single-to-noise ratio using widefield microscopy and high-sensitivity microscope cameras
- When needed, obtain higher resolution 3D images with spinning disk, confocal, or light sheet microscopy.
FAQs
The nematode, C. elegans, is a model organism used for studying genetic influences on aging, diseases, and lifespan. Also, they can be exploited for neuroscience due to the simple nervous system, behavioral dynamics like chemotaxis and thermotaxis, and screening drugs which may affect aging and disease.
Each hermaphroditic adult worm has 959 cells. The hermaphroditic worms can self-replicate. A worm can make 300 to 350 progenies during its lifetime. The large brood size, short life span, and variety of phenotypes makes the worms appropriate for genetic screening.
About 30 to 60% of genes in C. elegans have orthologs or functional homologs with mammalian genes. This fact implies that gene function in these worms may be relevant to human development and disease.
The transparency of the worm, the fact that every C. elegans has the same number of cells, and that each cell lineage and location has been mapped out. These are key advantages when using it as a model organism for development and disease studies.