The “Waffle Method”
High-pressure freezing (HPF) has become a cornerstone of cryo-electron tomography (cryo-ET), enabling the preservation of native biological structures with exceptional fidelity, especially in thick or complex specimens. For this, the sample is typically vitrified in a volume created by sample carriers up to 200 µm. Unfortunately, the large, vitrified volume must be specifically milled down in several dimensions to create a thin sheet, which then can undergo electron tomography.
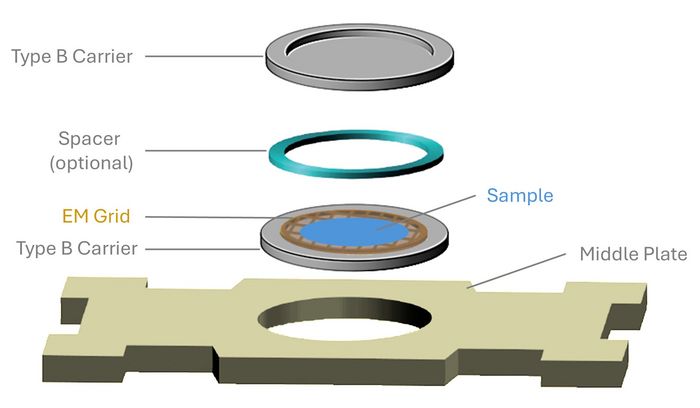
In particular for thicker specimens a method turned out to be more and more popular in the last years: The “Waffle Method”. The foundation for this approach was laid by McDonald et al. 2010 and further adjusted by Mahamid et al. in 2015, who first used a grid as a sample holder in a sandwich between the sample carriers (Fig 4). The term “Waffle Method” was then officially adopted in a 2022 study by Kelley et al., who standardized the technique and expanded its application across diverse biological systems.
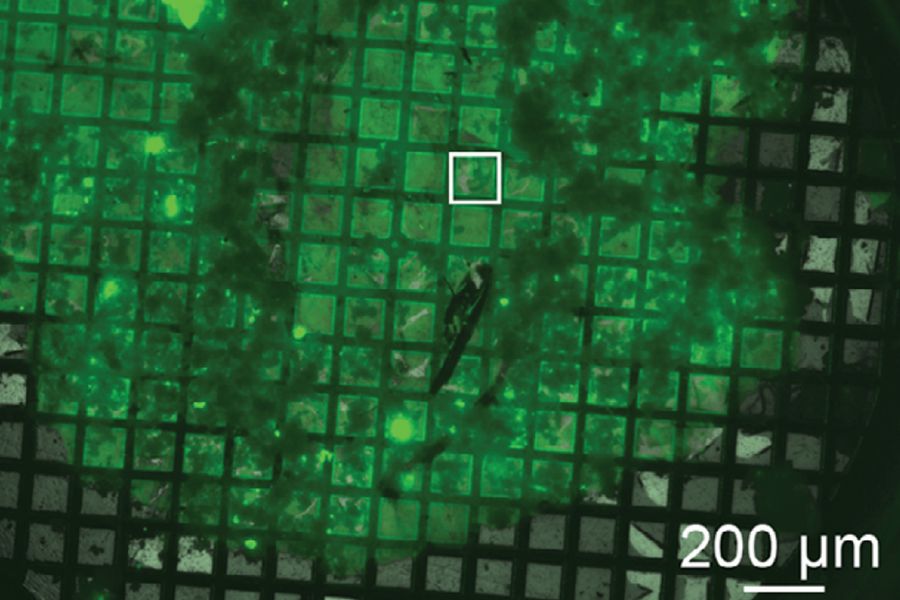
The technique has the advantage of creating a smaller volume for the subsequent FIB milling in contrast to standard HPF planchettes. The sample is applied into a grid, where the carbon coating functions as the bottom. This results in a vitrified “disc” contained by the grid after HPF with a thickness of about 20 – 30 µm instead of 100 – 200 µm as for standard planchettes. Hence, the amount of vitrified ice needed to be milled to access the sample in depth is massively reduced. Figure 1 shows a fluorescent mouse hippocampus brain slice on an EM grid after successful HPF (Matsui et al., 2024).
Aya Matsui, Cathy Spangler, Johannes Elferich, Momoko Shiozaki, Nikki Jean, Xiaowei Zhao, Maozhen Qin, Haining Zhong, Zhiheng Yu, Eric Gouaux (2024) Cryo-electron tomographic investigation of native hippocampal glutamatergic synapses eLife 13:RP98458; https://doi.org/10.7554/eLife.98458.3
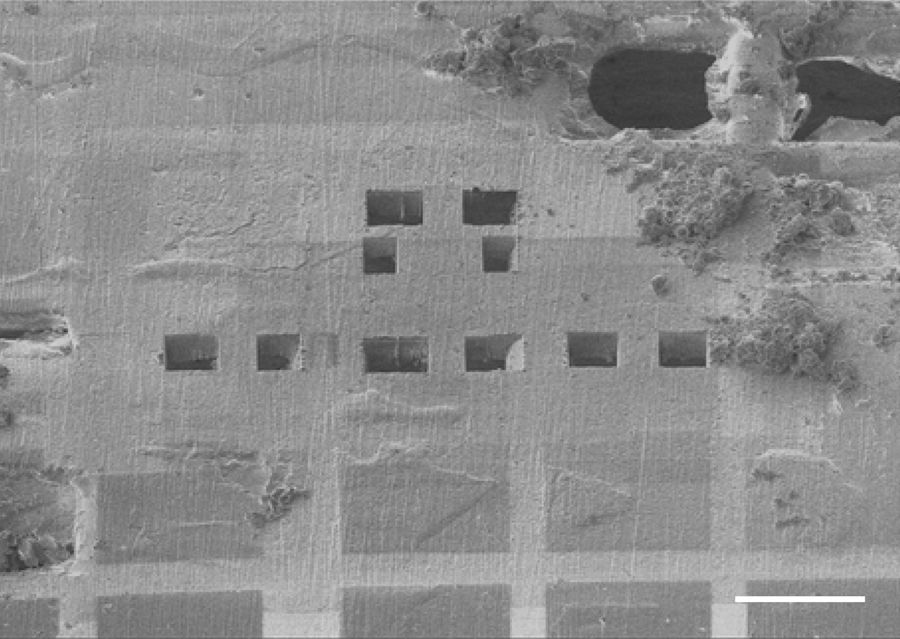
The grid bars stabilize the subvolumes in the grid squares for milling, particularly during the creation of trenches to approach the final thickness of 200 nm lamellae. In Figure 2 by Kelley et al. one can see the bright grid bars hidden under the surface in the lower area of the image. Above the grid bars, 5 pairs of trenches are visibly prepared for the subsequent fine milling of the target lamellae.
Kelley, K., Raczkowski, A.M., Klykov, O. et al. Waffle Method: A general and flexible approach for improving throughput in FIB-milling. Nat Commun 13, 1857 (2022). https://doi.org/10.1038/s41467-022-29501-3
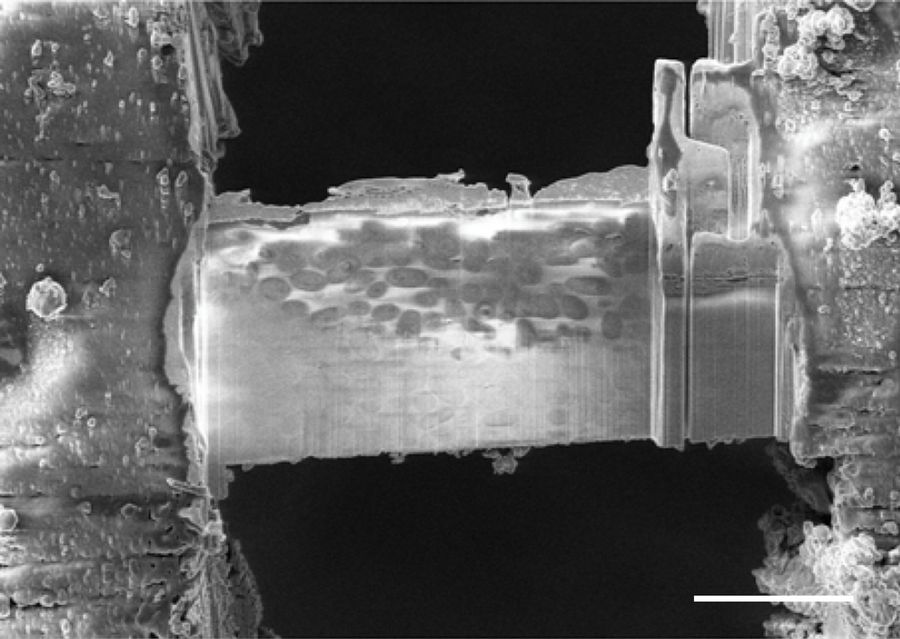
In Figure 3 an exemplary lamella is shown, hosting microsporidia in all orientations, which was achieved by the “Waffle Method”, but not using standard grid plunging techniques.
Kelley, K., Raczkowski, A.M., Klykov, O. et al. Waffle Method: A general and flexible approach for improving throughput in FIB-milling. Nat Commun 13, 1857 (2022). https://doi.org/10.1038/s41467-022-29501-3
In addition to the advantages mentioned above, the grid can be eventually mounted into a cryo-TEM holder, which is not possible with a standard but impenetrable planchette. This avoids sophisticated lift-out methods with risky steps of transferring lamellae into specific holders for the tomography in the cryo-TEM.
State-of-the-art automatic high-pressure-freezer: EM ICE
The EM ICE high-pressure freezer is particularly well-suited for the “Waffle Method” due to several key features that align with the method’s demands for precision, reproducibility, and compatibility with bulky or structured samples:
Compatibility with a grid as spacer
The ”Waffle Method” involves placing the sample in a grid sandwiched by two polished sample carriers. The EM ICE supports custom carriers and spacers according to the needs of the specimen, allowing for consistent sample thickness and optimal freezing geometry. Depending on the specimen size, different grid mesh types are used (90 µm up to 500 µm grid hole diameter, commonly square, but also in hexagonal shapes).
High Throughput and Reproducibility
The EM ICE is designed for repeatable freezing cycles, which is crucial when preparing multiple “Waffle“ samples for high-throughput cryo-FIB milling.
Minimal Sample Disturbance
The system allows for gentle handling and rapid transfer into liquid nitrogen, reducing the risk of mechanical damage or devitrification—especially important when working with delicate grid assemblies.
Use the waffle method now as well with membrane carriers. The proof of principle using the EM ICE with membrane carriers was done with baker's yeast.
Integration with Downstream Cryo-Workflows
The EM ICE is widely used in workflows that include cryo-FIB/SEM and cryo-ET, making it a natural fit for the “Waffle Method”, which relies on an effective workflow from freezing to milling to imaging.
The EM ICE used for the “Waffle Method”
Recently, the sample regime using the “Waffle Method” was expanded to include plant tissue by Pöge et al. (elifeSciences). Tissues from moss, Arabidopsis thaliana and Limonium bicolor (sea lavender) were used.
In Nature Methods, Schiøtz et al. present Serial Lift-Out, a technique that combines HPF with high-throughput cryo-FIB milling to extract multiple lamellae from a single sample. This enables volumetric sampling of multicellular organisms like C. elegans, while maintaining ultrastructural integrity across large tissue regions.
In eLife, Matsui et al. apply HPF to mouse brain tissue, integrating it with cryo-correlative light and electron microscopy to study native hippocampal synapses. Their approach ensures rapid vitrification of neural circuits, allowing for precise localization and structural analysis of AMPA receptors in situ.
Berger et al. (bioRxiv) demonstrate that HPF is compatible with xenon plasma FIB milling, enabling high-throughput preparation of lamellae from thick samples. Their work achieves near-atomic resolution reconstructions, such as the E. coli ribosome at 4.0 Å, underscoring HPF’s critical role in preserving macromolecular architecture.
Acknowledgements
This application note shows images of the following articles under a Creative Commons License (Attribution 4.0 International, as described at CreativeCommons.
Waffle Method: A general and flexible approach for improving throughput in FIB-milling.
Kelley, K., Raczkowski, A.M., Klykov, O. et al.
Nat Commun 13, 1857 (2022).
Cryo-electron tomographic investigation of native hippocampal glutamatergic synapses
Aya Matsui, Cathy Spangler, Johannes Elferich, Momoko Shiozaki, Nikki Jean, Xiaowei Zhao, Maozhen Qin, Haining Zhong, Zhiheng Yu, Eric Gouaux
eLife 13:RP98458 (2024).