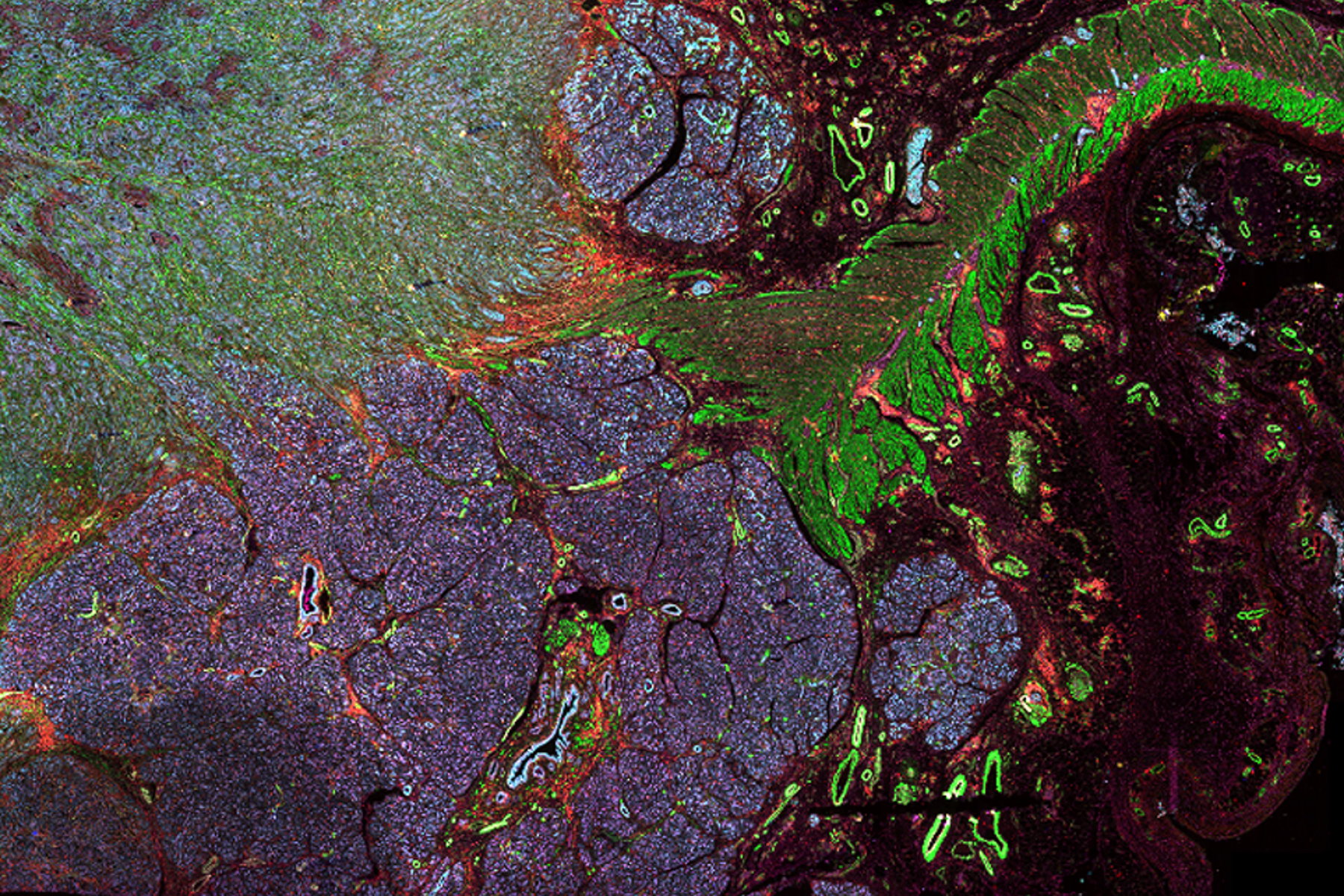
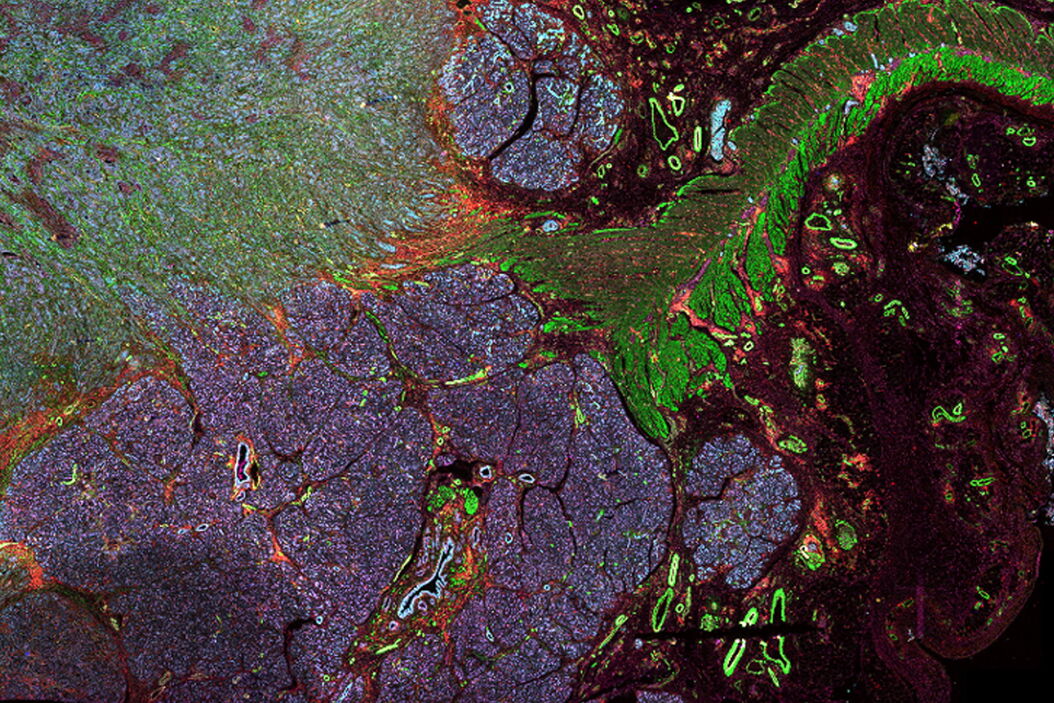
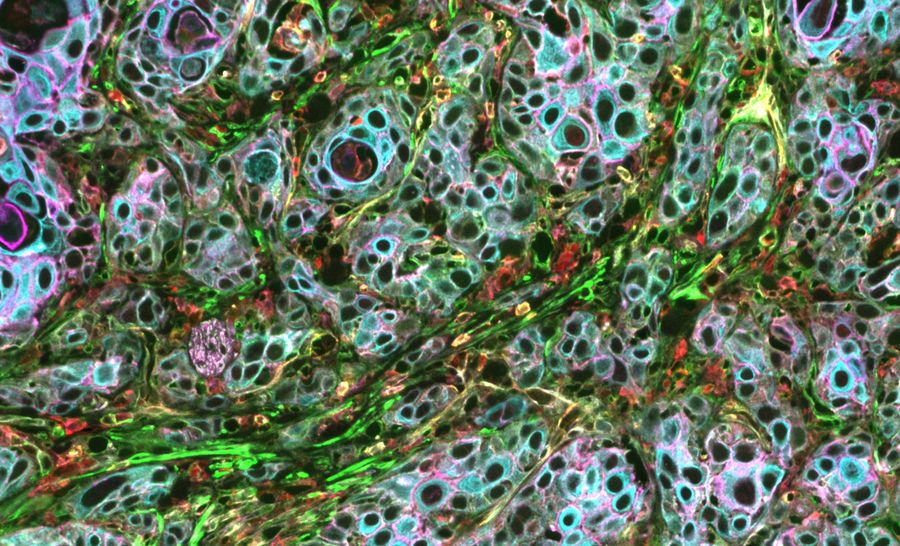
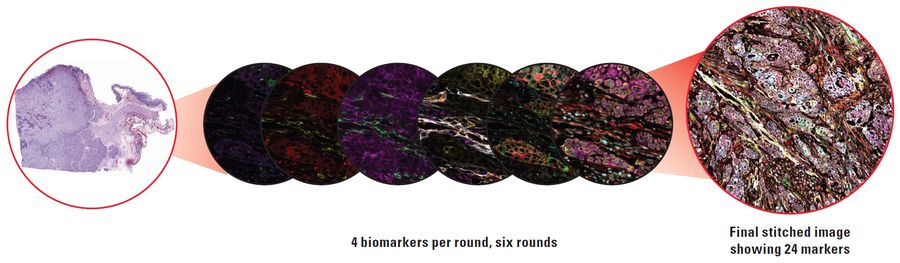
In this article, we explore the different types of multiplexing technologies available, as well as their benefits and disadvantages. Specifically, we cover the main categories of multiplexed imaging: staining and imaging in one, one-pass lower-plex, all-in-one omics solutions, and iterative staining. We also compare these technologies based on their number of biomarkers, ease of use, multiple slides, biomarker selection flexibility and resolution.
Additionally, we provide technical information, pros and cons, and background information so you can have a foundation to understand the current multiplexing landscape. Finally, we describe how Cell DIVE, our open multiplex solution, fits into the rapidly evolving multiplexing space, and resolves many of the pitfalls and challenges involved in executing a study.
Introduction
Multiplexed immunofluorescence imaging is a relatively new, exciting, and rapidly growing technique in life science research. If you perform tissue research and ever wished that you could get more information out of a single sample, consider adding multiplexed imaging to your toolbox. At the most basic level, multiplexed immunofluorescence is a term that describes techniques that allow you to probe a single sample with more biomarkers than possible in a traditional fluorescence experiment (6 – 60+).
For researchers that are new to this space, or are just beginning to investigate multiplexing, it can be difficult to understand the different types of solutions that are available and even more difficult to make a final choice of solution. Read on to learn what factors to consider during your multiplexed imaging solutions evaluation, how each solution might fit with your research, and the limitations that each approach brings.
Staining and imaging in one
For this category of multiplexing systems, the staining process is performed automatically using perfusion or microfluidics. The stainer can be integrated into the imager itself or is separate from the imager but closely associated. The draw of this type of multiplexing solution is its ease of use – oftentimes reagent panels are kitted, reducing the effort required for study design, and it is possible to complete a multiplexing experiment for a single slide with minimal hands-on time.
Because of the small capacity of this type of solution, samples must be processed one at a time or in very small batches (< 3 slides). With small study cohorts, this can be acceptable, but as research evolves to address larger cohorts (10s to 100s of slides), slides from the same study may be processed weeks or even months apart, introducing variability that could affect results. Additionally, since many of these all-in-one solutions utilize kitted reagents, flexibility with respect to biomarker panels can be limited.
One-pass lower-plex
These systems are lower-plex because they can image 9-12 biomarkers, but they do so in a single pass of staining and imaging, usually using spectral unmixing techniques. Since these systems are single-pass, they are very scalable and can process many slides quickly in a straightforward fashion. Additionally, for the limited number of biomarkers that can be interrogated, custom panels can be designed
reasonably easily.
The downfall of the one-pass lower-plex techniques is the low number of imaged biomarkers. You may be able to image hundreds of slides but with only 9-12 biomarkers you will not acquire a comprehensive picture of the tissue microenvironment that higher-plex techniques offer.
All-in-one omics solutions
At the opposite end of the spectrum in terms of plexity are solutions in which an omics technology (mass spectrometry or nucleic acid sequencing) is used to extract information from regions of tissue. In these solutions, regions of tissue can be excised from the whole piece and processed to yield protein or nucleic acid expression data. These solutions offer the highest degree of plexity and can potentially analyze thousands of biomarkers at once.
However, these solutions have two major drawbacks. First, the removal of regions of tissue for mass spectrometry or RNAseq destroys those regions. Tissue analyzed with these technologies cannot be returned to for later imaging or future questions. This can be especially difficult if precious tissues from patients are being studied. Second, while the biomarker expression data can be mapped back to an image of the tissue to add spatial context to expression profiles, the overall resolution of this mapping is lower than for technologies which use direct, fluorescent imaging.
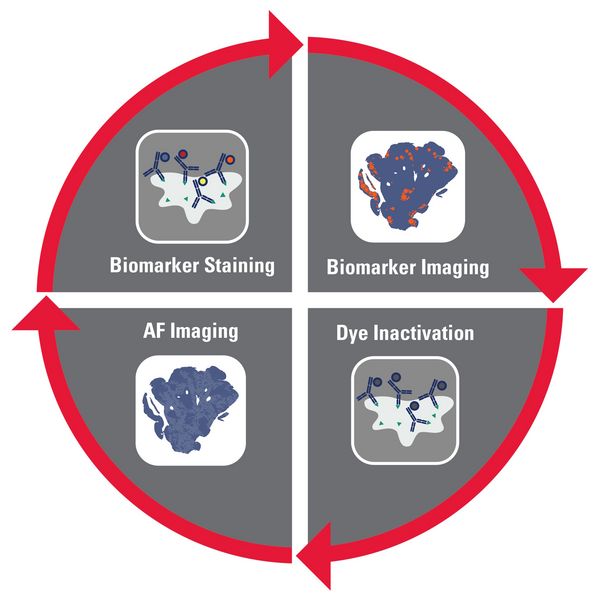
Iterative staining
As the name suggests, iterative staining multiplexing techniques requires samples to be stained and de-stained in a cyclic process. In each cycle, new biomarkers are used resulting in a final image with many biomarkers, although typically under 100. In an iterative staining workflow, staining occurs outside the imager, so it lends itself to processing slides in batches using automated staining techniques. This allows large study cohorts to be processed through an iterative staining workflow together. Iterative staining techniques tend to be very flexible with respect to the biomarker selection – choose how many biomarkers to use, which biomarkers to use, and where to source them – the choice is yours.
The scalability and flexibility of iterative staining techniques does have a cost though. During each cycle, the sample must be accessed twice – once each for staining and de-staining – and imaged twice. Using a traditional coverslipping approach, this means that a sample would have to be coverslipped and decoverslipped twice for each cycle. The coverslipping and decoverslipping process can damage precious tissue samples and significantly impacts the ease of use of iterative staining techniques.
Why can’t we have the best of ALL of this?
As with so many things, compromise has historically been required when choosing a multiplexing method. When designing your study, consider the following: do you need ease of use or scale? Or alternatively, do you need many slides or many markers? Finally, can your solution keep up with the evolution of your study? Is tissue preservation important?
Unfortunately, your research is what dictates what compromises are acceptable and in the multiplexing space, it is increasingly clear that compromise is the enemy of progress.
Compairing available multiplexing techniques
| Staining and imaging in one | One-pass lower plex | Iterative staining | All-in-one omics | Cell DIVE and ClickWell | |
|---|---|---|---|---|---|
| No. of biomarkers | + | + | + | ++ | |
| Ease of use | + | + | + | ||
| Multiple slides | + | + | + | ||
| Biomarker selection flexibility | + | + | + | + | |
| Resolution | + | + | + | + |
Table: Compairing available multiplexing techniques
How can we do better?
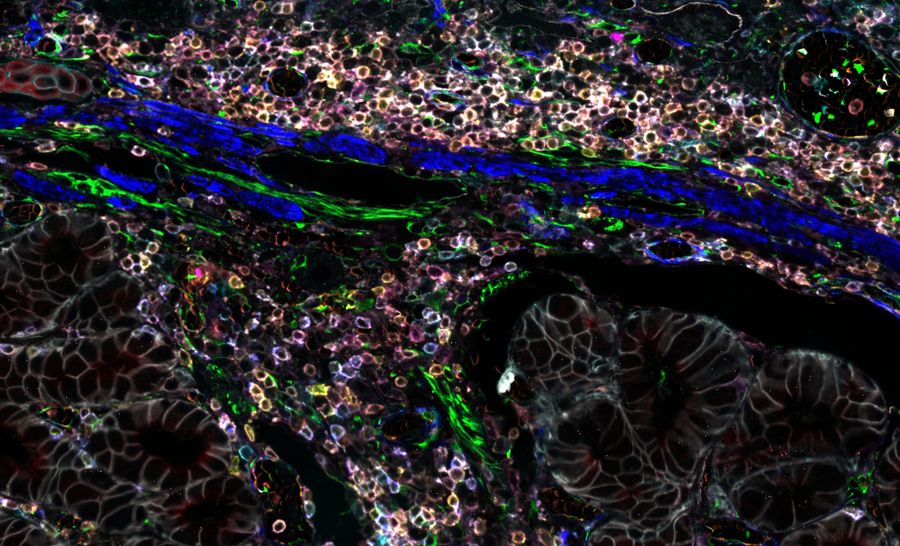
When designing your study, it is crucial to find a multiplexed imaging solution that adapts to your research needs. The Cell DIVE multiplexed imaging solution is purpose-built and easy to scale system designed specifically to simplify multiplexed imaging. Based on an iterative staining process, Cell DIVE produces clear tissue imaging of 60+ biomarkers. Cell DIVE allows you to extend the life of your precious samples via an innovative coverslip-free workflow and gentle deactivation procedure. This same workflow enables you to reduce sample processing time, giving you time back to focus on insight creation.
Cell DIVE resolves many of the technical challenges inherent to other multiplexed imaging techniques. The iterative staining approach resolves the limited plexity of one-pass approaches, and antibody testing and validation pre-work is minimized through an included list of 350+ pre-validated antibodies. The main pain point of other iterative staining approaches, repetitive coverslipping and decoverslipping, is eliminated by the ClickWell slide carrier.
ClickWell is an innovative new slide carrier that allows staining and dye inactivation solutions to be applied without placing or removing coverslips, ensuring minimal tissue damage over many rounds of imaging.
In addition, due to ClickWell’s the form factor, Cell DIVE can easily be paired with robotic plate loaders to add multiple ClickWell plates to the imager without human intervention. This compatibility allows the researcher to radically increase the scale and efficiency of their experiment. The precise placement of the slide into the carrier by the cams also allows the user to remove slides as desired for staining outside of the ClickWell, unlocking a host of staining methods to fit any labs expertise.
Cell DIVE, especially when paired with ClickWell, is an open multiplex imaging solution that delivers high quality images, automation, and accurate insights into the biology of your tissue. While the landscape of multiplexed imaging options is complex and changing rapidly, Cell DIVE offers you key advantages and ensures the generation of accurate, reproducible data.