Fluorescent Proteins from A. Victoria - What is green fluorescent protein (GFP)?
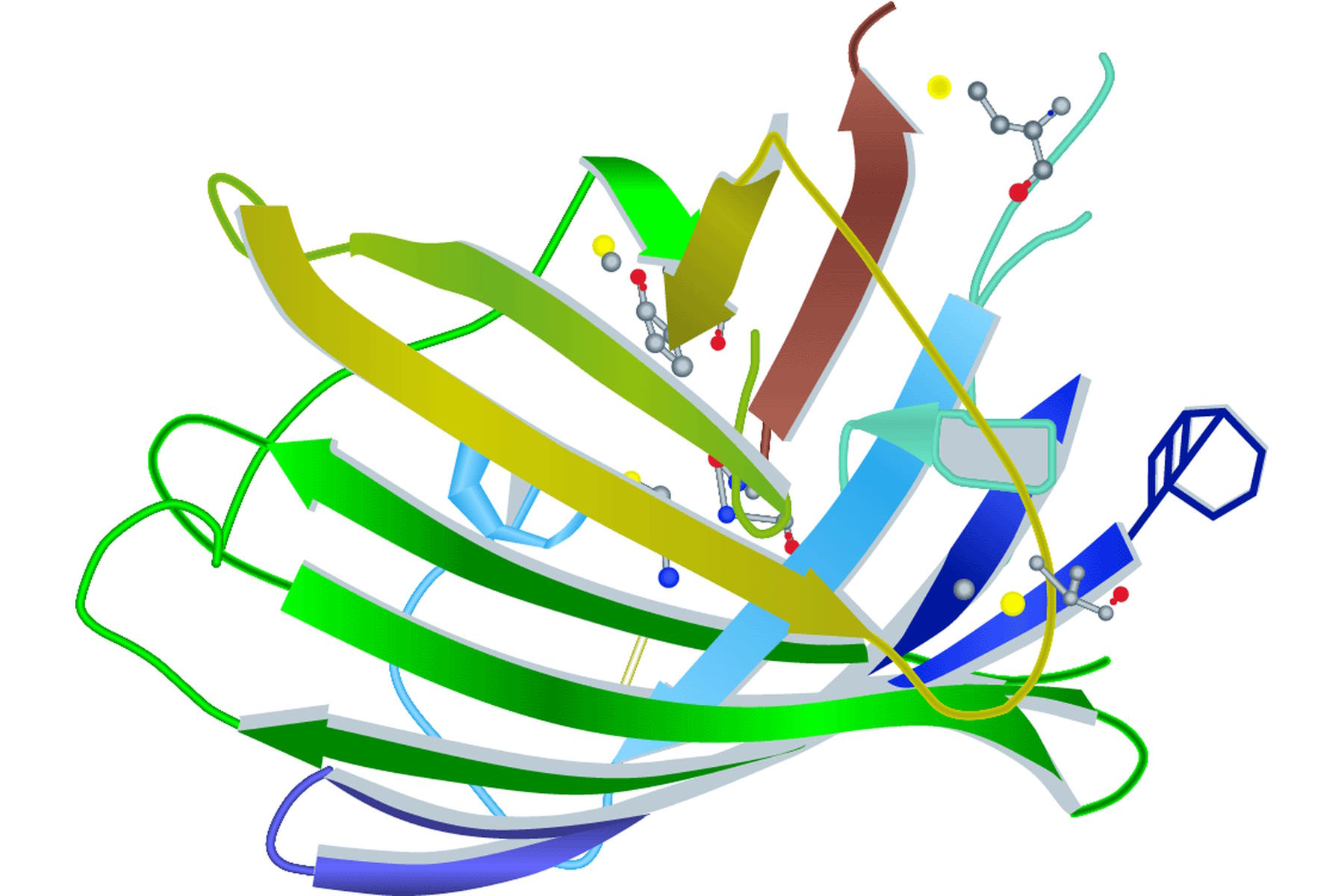
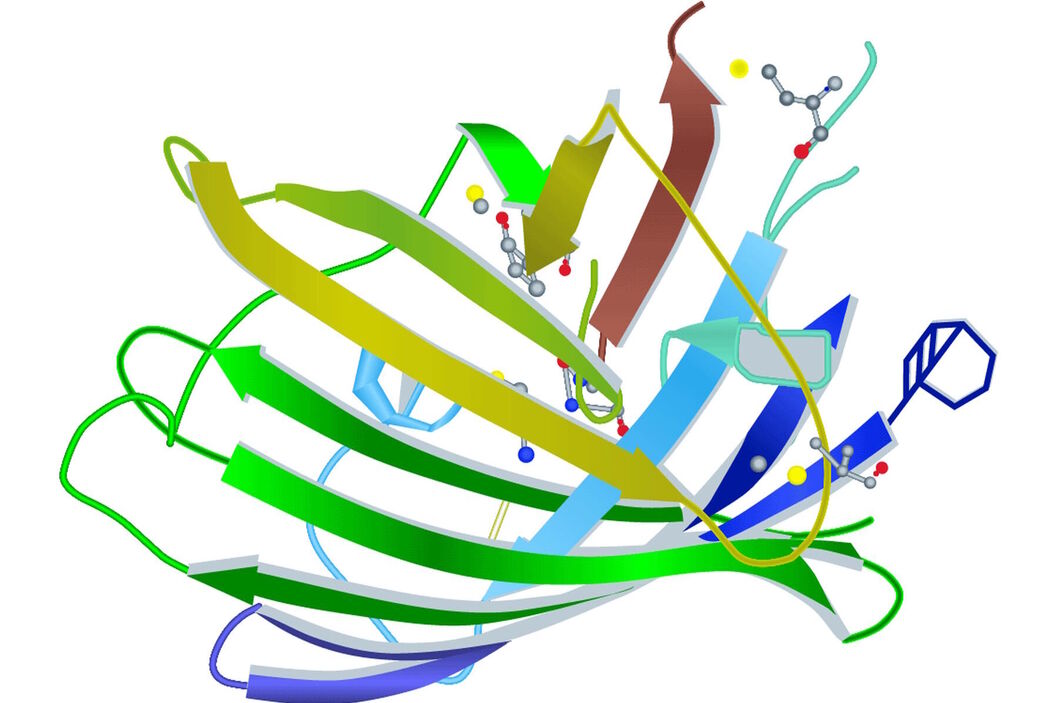
Spectral properties of GFP (green fluorescent protein) or its variants lie in the amino acid structure forming the chromophore (Figure 1) [2]. It can be the three amino acids at positions 65–67 or residues close to this location (e.g. YFP). Besides the main mutations concerning the chromophore, research was also conducted on other site-directed mutagenesis to improve other factors like protein maturation and expression in heterologous cell systems (e.g. codon usage, protein folding at physiological temperature). Note that A. victoria is a relatively primitive ectotherm marine organism with no physiological system to regulate its body temperature.
Even if GFP is one of the most popular FPs, because of its brightness and high photostability, it has two main disadvantages: a certain sensitivity to pH and slight tendency to dimerize. Dimerization or oligomerization is a problem of many FPs. Their preference to agglutinate with each other can produce artifacts or misinterpretations concerning location and function of the fused protein. However, scientists came up with some answers to that problem too. Mutations at critical positions (F223R, L221K and A206K) where non-polar amino acids are replaced by hydrophilic ones show reduced dimerization [3]. All the genetic changes which lead to an improvement of spectral as well as practical characteristics are summarized under the name of "enhanced" FPs.
In the case of wtGFP, enhancements lead to an EGFP (enhanced GFP) with a single excitation peak at 488 nm instead of the previous complex absorption spectrum at 395 nm and 475 nm [2,3]. The first mutated version of wtGFP (the S65T mutant) developed by Roger Heim, Douglas Prasher, and Roger Tsien was five times brighter than the original and showed a shorter maturation time [3]. Together with better maturation efficiency at 37°C, based on another mutation (F64L), this plays an important role for people looking at living cells.
A very interesting GFP variant with one of the largest Stokes shifts is Sapphire [3]. A mutation at a position close to the chromophore (T203I) leads to an alteration of the excitation maximum to 399 nm and the emission maximum to 511 nm. This is a Stokes shift of 112 nm. Emerald is another GFP modification with improved photostability and brightness and more efficient folding in mammalian cells [3].
Whereas all the green fluorescent proteins have a relatively high brightness, blue fluorescent proteins normally suffer from reduced emission intensity in microscopic applications. Nevertheless, they are used in optical assays because of other spectral properties. EBFP (Enhanced Blue Fluorescent Protein) was constructed by several rounds of mutating wtGFP [3]. The first one (Y66H) shifted the emission peak from green to blue. More mutations followed, producing a protein with an excitation maximum at 380 nm and an emission maximum at 448 nm. These spectral properties make it a partner for EGFP in FRET microscopy. More recent blue fluorescent proteins with higher quantum yields and better photostability are Azurite, SBFP2 and EBFP2 [3]. A promising EBFP successor is a protein named Sirius which became popular because of its very high tolerance to pH (stable from pH 3 to 9) and its reputation for being the fluorescent protein with the shortest emission wavelength to date [3].
A second “blue” class of GFP variants is formed by cyan fluorescent proteins (CFPs) [3]. Substitution of tyrosine with tryptophan (Y66W) and further genetic alterations lead to a fluorochrome with improved brightness and photostability. This ECFP has a bimodal excitation and emission spectrum at 433/445 nm and 475/503 nm. Brightness is only about 40% of that of EGFP. A prominent ECFP variant is Cerulean, which has a higher extinction coefficient and quantum yield [3]. It is 1.5 times brighter than ECFP and is used as a FRET partner with YFP.
A GFP mutation which does not directly alter one of the three central amino acids in the chromophore resulted in the rise of yellow fluorescent proteins (YFPs) [3]. YFPs have a common threonine at position 203 which is exchanged by a tyrosine (T203Y). This amino acid is part of the β-barrel and lies in close proximity to the chromophore. Compared to GFP, excitation and emission properties have been shifted to longer wavelengths with excitation and emission maxima at 514 nm and 527 nm (EYFP). One characteristic of EYFP is its pH sensitivity. At pH 6.5, EYFP has only about 50% of its fluorescence, which is not always a disadvantage. When it comes to pH measurement (e.g. vesicles, endosomes, etc.), EYFP can be used as an indicator. Interestingly, a further mutation (Q69M) developed a better acid stability and dramatically improved brightness (75% brighter than EGFP). This protein, which still has a poor photostability compared to EGFP, was called Citrine [3]. Another YFP mutant (F46L) showed a drastically faster maturation speed and also an improved pH resistance. This protein was named Venus and is a frequent FRET acceptor with Cerulean [3].
Fluorescent Proteins from Anthozoa
As one can see from above, most FPs coming from the jellyfish A. victoria emit light in the blue to the yellow range of the spectrum. Red fluorescing proteins are missing. The Russian scientist Sergey Lukyanov closed the gap when he discovered FPs in Anthozoa [4]. Red fluorescent proteins (RFPs) have a big advantage over others, because there is much less autofluorescence in the cells in the red part of the spectrum [3]. Furthermore, they are excited by longer wavelengths, which is better for living cells, as light of shorter wavelengths does far more damage to specimens. A further general advantage of coral proteins over jellyfish proteins is their efficient maturation at 37°C. Whereas A. victoria GFP and derivatives had to be genetically modified to fold in the right way, Anthozoan proteins mature without the necessity of molecular engineering. This fact might be due to the warmer water temperatures of their biotope.
The first and still one of the most used FPs discovered in Anthozoa is DsRed [3]. The name is derived from the sea anemone Discosoma striata. DsRed has an excitation maximum at 558 nm and an emission peak at 583 nm. However, the initial euphoria stagnated when its structural information was published. DsRed maturates much more slowly than jellyfish FPs and has an intermediate chromophore stage. This stage emits light in the green spectrum and causes overlap with other FPs. As already mentioned in the GFP section, DsRed also has another problem. The red fluorescing sea anemone protein is an obligate tetramer with the predisposition to form oligomers. This can lead to misinterpretations concerning location and function of the fused protein. In general Anthozoa FPs have a similar structure to that of A. victoria FPs. The chromophore is hidden in a β-barrel structure with the size of 4 nm x 3 nm (height x diameter). The difference lies in the more elliptical appearance of the Anthozoa β-barrel (Figure 2).
In parallel to GFP "evolution", researchers started to modify the original DsRed to overcome its structural deficiencies. The second DsRed generation – DsRed2 – has a reduced oligomer formation tendency and a faster maturation speed, minimizing the green light emitting intermediate stage [3]. Further mutagenesis led to a red FP which had totally lost its tetramer status, but also forfeited some of its quantum yield (25% of DsRed2). This work of the Tsien laboratory resulted in the first monomeric red fluorescent protein, so they called it mRFP1 [3].
This mRFP1 was then the starting point for the creation of a group of six monomeric FPs collectively called "mFruit" [3]. Their individual names are derived from their emission color: mHoneydew, mBanana, mOrange, mTangerine, mStrawberry and mCherry. mCherry is the most useful of these FPs, emitting light in the range of 610 nm with a brightness which is 50% that of EGFP.
The brightest FP so far is a follower of the mFruit faction and has the name tdTomato [3]. Until it was genetically changed, dTomato was an obligate dimer, but dimerization was avoided by putting the two dimerization partners in one molecule. Two dTomato units were coupled by a 12 amino acid linker creating the tandem dimer FP tdTomato with an emission maximum at 581 nm and photostability in the highest region of the spectrum.
The emission spectrum was shifted further into the far red (630 nm – 700 nm) when another mFruit successor was created. mPlum is the mFruit member with the deepest red emission of any of the mFruit proteins at 649 nm [3].
The amount of scientifically used green fluorescing Anthozoan proteins is very small, which is not very surprising in view of the availability of the well-known and user-friendly A. victoria GFP. Obviously, no one saw any necessity to establish a new green fluorescent protein. Nevertheless, there are some, like a bright fluorescent protein from the stony coral Galaxeidae, Azami Green [3]. Interestingly, its sequence homology with EGFP is less than 6%.
An anthozoan FP which has had a high impact on deep tissue imaging is Katushka [3]. Applying mutagenesis to RFPs coming from E. quadricolor, Katushka was identified as a dimeric protein with an emission maximum at 635 nm and the highest brightness levels of all deep-red fluorescing proteins. The monomeric form of Katushka is called mKate and was supplied with a higher brightness later on, resulting in mKate2 [3].
In summary, all the fluorescent proteins which are used today for microscopic applications were derived from primitive marine creatures. Table 1 comprises the most important ones together with their relevant spectral characteristics like excitation and emission maxima, photostability, quantum yield, and brightness.
Outlook
What turned out to be a very interesting story was the discovery of a FP expressed by a vertebrate. Amphioxus, a small fish-like marine chordate, produces AmphiGFP at its anterior side [3]. The sequence analysis of that FP predicts a typical β-barrel structure and seems to be related to CopGFP of the copepod Pontellina plumata (Crustacea) [5]. This finding showed that the phenomenon of fluorescence is not restricted to primitive invertebrates, but can also be found in animals of higher evolutionary stages. Furthermore, this discovery shows the ongoing process of fluorescent-protein discovery, modfication, and enhancement which are still popular research topics. This fact corroborates the importance and high impact fluorescent proteins have for current and and will continue to have for future life-science research.
Spectral Characteristics of Fluorescent Proteins
- Ex: Peak excitation wavelength (nm)
- Em: Peak emission wavelength (nm)
- MW: Molecular weight QY: Quantum yield
- BR: Brightness;
- Extinction coefficient * Quantum yield/1,000
- PS: Photostability; time to 50% brightness (sec)
Fluorescent Proteins | ||||||||
Ex (nm) | Em (nm) | MW | QY | BR | PS | Source | Other | |
Y66H | 360 | 442 | ||||||
Y66F | 360 | 508 | ||||||
EBFP | 380 | 440 | 0.18 | 9 | Addgene | Monomer | ||
EBFP2 | 383 | 448 | 0.56 | 18 | Addgene | Monomer | ||
Azurite | 383 | 447 | 0.55 | 14 | Monomer | |||
GFPuv | 385 | 508 | ||||||
T-Sapphire | 399 | 511 | 0.60 | 26 | 25 | Weak dimer | ||
402 | 457 | 26 k | 0.63 | 33 | ++ | Evrogen | Monomer | |
Cerulean | 433 | 475 | 0.62 | 27 | 36 | Weak dimer | ||
mCFP | 433 | 475 | 0.40 | 13 | 64 | Monomer | ||
ECFP | 434 | 477 | 0.15 | 3 | ||||
CyPet | 435 | 477 | 0.51 | 18 | 59 | Weak dimer | ||
Y66W | 436 | 485 | ||||||
dKeima-Red | 440 | 616 | 0.31 | 8 | MBL | Dimer | ||
mKeima-Red | 440 | 620 | 0.24 | 3 | MBL | Monomer | ||
458 | 480 | 0.57 | 29 | Evrogen | Dimer | |||
458 | 489 | 0.75 | 29 | Clontech | Tetramer | |||
mTFP1 (Teal) | 462 | 492 | 0.85 | 54 | Dimer | |||
S65A | 471 | 504 | ||||||
Midoriishi-Cyan | 472 | 495 | 0.9 | 25 | MBL | Dimer | ||
Wild Type GFP | 396,475 | 508 | 26 k | 0.77 | 16 | |||
S65C | 479 | 507 | ||||||
482 | 502 | 26 k | 0.53 | 37 | Evrogen | Dimer | ||
482 | 505 | 27 k | 0.59 | 34 | ++ | Evrogen | Monomer | |
483 | 506 | 27 k | 0.6 | 34 | ++ | Evrogen | Monomer | |
484 | 510 | 27 k | 0.82 | 27 | Clontech | |||
S65L | 484 | 510 | ||||||
Emerald | 487 | 509 | 0.68 | 39 | 0.69 | Invitrogen | Weak dimer | |
S65T | 488 | 511 | ||||||
EGFP | 488 | 507 | 26 k | 0.60 | 34 | 174 | Addgene | Weak dimer |
Azami-Green | 492 | 505 | 0.74 | 41 | MBL | Tetramer (monomeric available) | ||
493 | 505 | 105 k | 0.91 | 40 | Clontech | Tetramer | ||
Dronpa-Green | 503 | 518 | 0.85 | 81 | MBL | Photoswitchable | ||
508 | 524 | 27 k | 0.62 | 47 | Evrogen | Monomer | ||
EYFP | 514 | 527 | 26 k | 0.61 | 51 | 60 | Weak dimer | |
Topaz | 514 | 527 | 0.60 | 57 | Monomer | |||
Venus | 515 | 528 | 0.57 | 53 | 15 | Weak dimer | ||
mCitrine | 516 | 529 | 0.76 | 59 | 49 | Monomer | ||
>YPet | 517 | 530 | 0.77 | 80 | 49 | Weak dimer | ||
525 | 538 | 26 k | 0.53 | 56 | Evrogen | Dimer | ||
525 | 537 | 26.8 k | 0.40 | 52 | ++ | Evrogen | Weak dimer | |
525 | 537 | 26.8 k | 0.39 | 48 | ++ | Evrogen | Monomer | |
529 | 539 | 0.65 | 13 | Clontech | Tetramer | |||
540 | 553 | 0.70 | 4 | Clontech | Monomer | |||
Kusabira-Orange | 548 | 559 | 0.60 | 31 | MBL | Monomer | ||
mOrange | 548 | 562 | 0.69 | 49 | 9 | Monomer | ||
549 | 565 | 0.60 | 35 | Clontech | Monomer | |||
mKO | 548 | 559 | 0.60 | 31 | 122 | Monomer | ||
553 | 574 | 26 k | 0.67 | 62 | Evrogen | Dimer | ||
554 | 581 | 0.69 | 95 | 98 | Clontech | Tandem dimer | ||
554 | 591 | 0.42 | 15 | Clontech | ||||
555 | 584 | 27 k | 0.48 | 48 | Evrogen | Monomer | ||
557 | 592 | ~28 k | 0.1 | 3.5 | 16 | Clontech | Monomer | |
563 | 582 | ~110 k | 0.55 | 24 | Clontech | |||
574 | 596 | 0.29 | 26 | 15 | Clontech | Monomer | ||
574 | 602 | 26 k | 0.35 | 26 | Evrogen | Dimer | ||
576 | 592 | ~110 k | 0.21 | 13 | Clontech | Tetramer | ||
mRFP1 | 584 | 607 | ~30 k | 0.25 | Tsien lab | Monomer | ||
J-Red | 584 | 610 | 0.20 | 8.8 | 13 | Dimer | ||
587 | 610 | 0.22 | 16 | 96 | Clontech | Monomer | ||
588 | 618 | ~52 k | 0.03 | 0.6 | Clontech | Dimer | ||
588 | 633 | 26 k | 0.40 | 25 | + | Evrogen | Monomer | |
588 | 635 | 26 k | 0.34 | 22 | ++ | Evrogen | Dimer | |
588 | 635 | 0.30 | 15 | Evrogen | Monomer | |||
588 | 635 | 26 k | 0.34 | 22 | Evrogen | Dimer | ||
590 | 649 | 0.10 | 4.1 | 53 | Clontech | |||
598 | 625 | 0.15 | 13 | Clontech | Monomer; faster photobleach than mPlum | |||
mNeptune | 600 | 650 | 0.20 | 13 | Tsien Lab | Monomer | ||
E2-Crimson | 611 | 646 | 0.23 | 29 | Clontech |
| ||
Table 1: Spectral characteristics of fluorescent proteins (data source is reference 6).
To find out more about fluorescent proteins and dyes, refer to these articles: