Zorkau M., Albus C., Berlinguer-Palmini R., Chrzanowska-Lightowlers Z. & Lightowlers R.
High-resolution imaging reveals compartmentalization of mitochondrial protein synthesis in cultured human cells
PNAS February 9, 2021 118 (6) e2008778118;
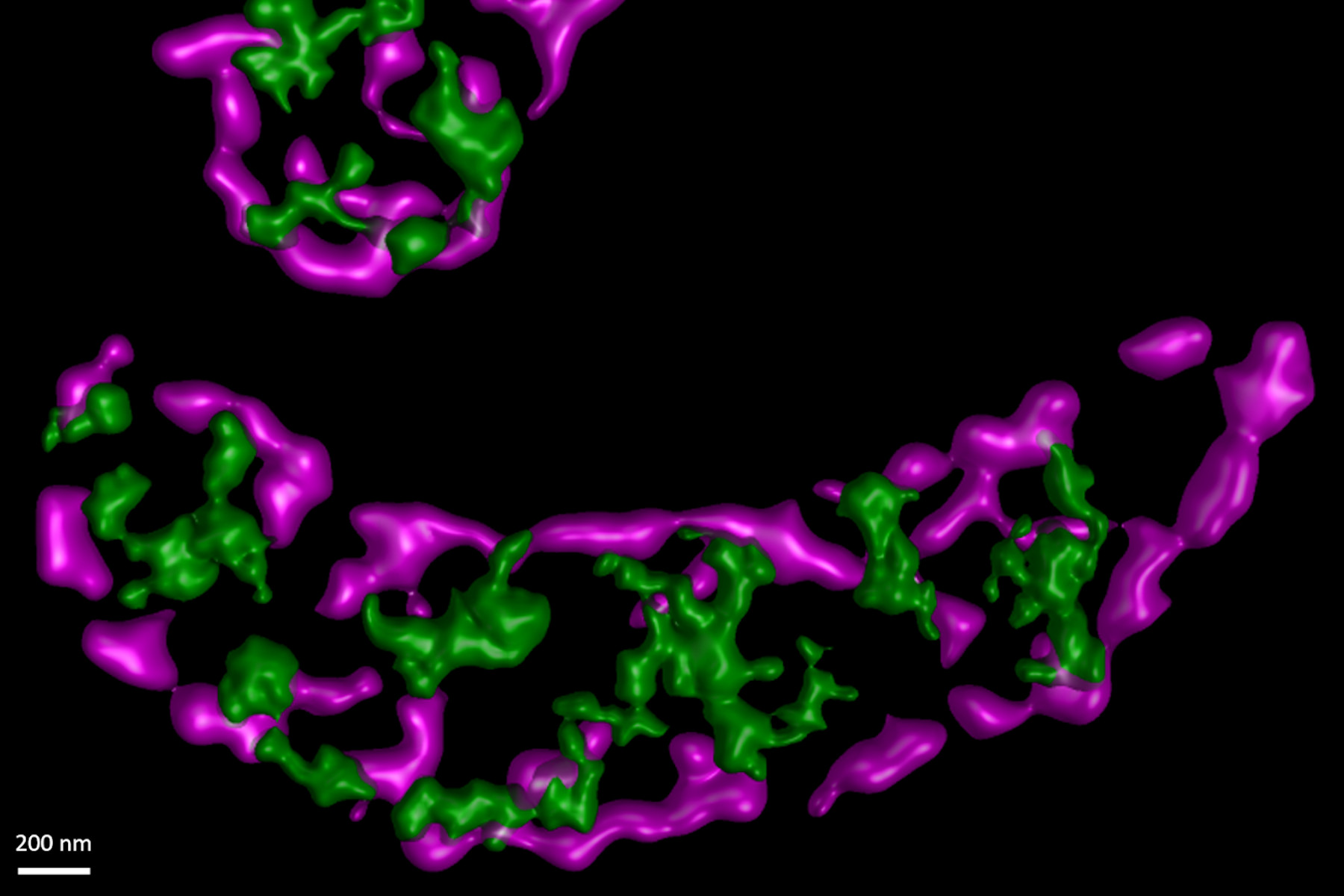
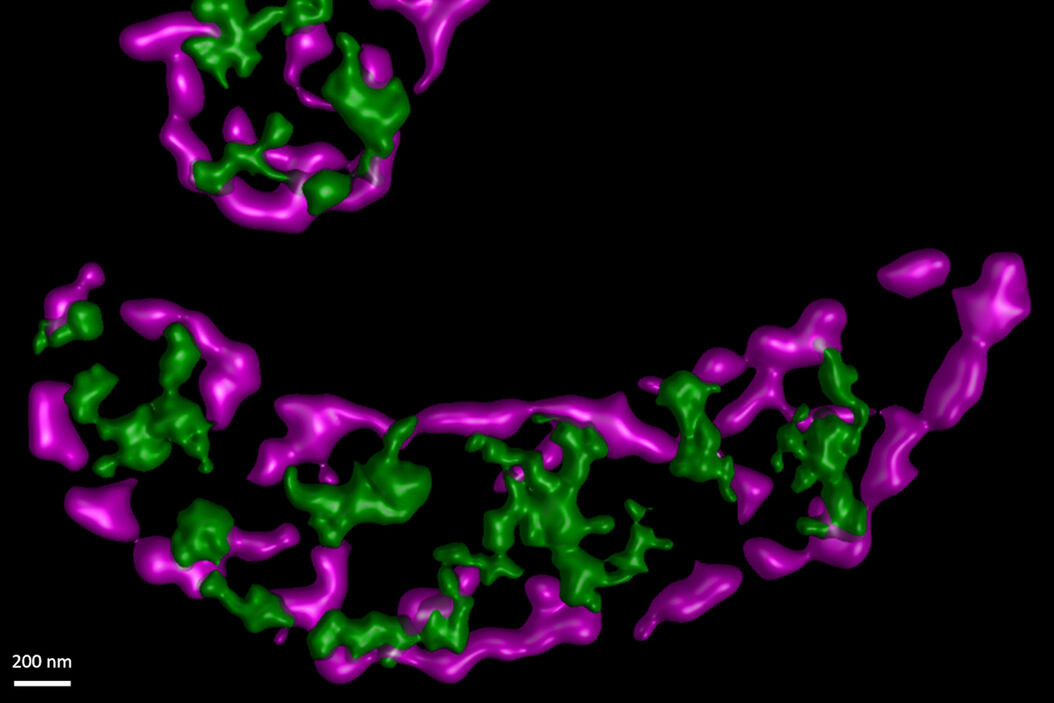
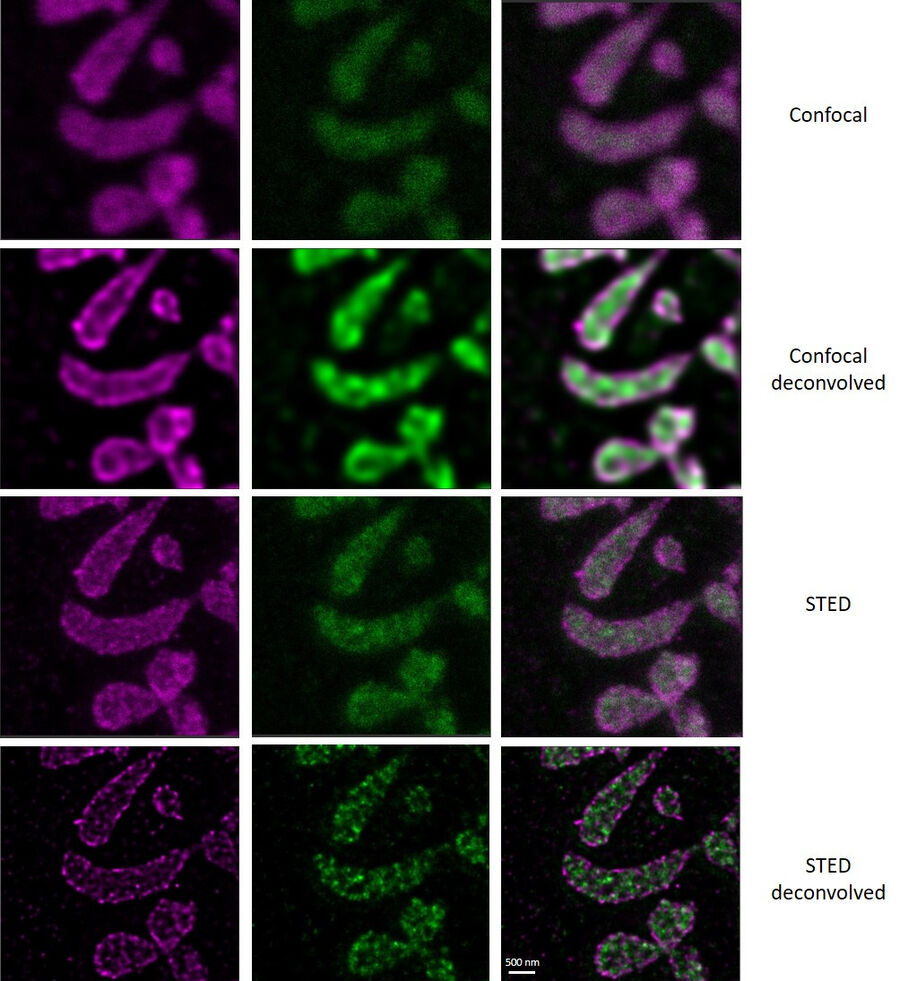
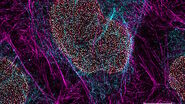
In this paper the authors have adapted a click chemistry-based method coupled with stimulated emission depletion nanoscopy (STED) to address these questions reporting that, in human cells in culture, the majority of mitochondrial protein synthesis is detected at the cristae membranes and is spatially separated from the sites of RNA processing and maturation.
(Deconvolved and rendered with Huygens – www.svi.nl)
Related Articles
-
Extended Live-cell Imaging at Nanoscale Resolution
Extended live-cell imaging with TauSTED Xtend. Combined spatial and lifetime information allow…
Mar 05, 2024Read article -
The Guide to STED Sample Preparation
This guide is intended to help users optimize sample preparation for stimulated emission depletion…
Mar 05, 2024Read article -

Five-color FLIM-STED with One Depletion Laser
Webinar on five-color STED with a single depletion laser and fluorescence lifetime phasor…
Dec 14, 2022Read article