Zebrafish Research
Taking substantiated decisions in Zebrafish research. For the best result during screening, sorting, manipulation, and imaging you need to see details and structures to make the right decisions for your next steps in research. Known for outstanding optics and superb resolution, stereo microscopes and transmitted light bases from Leica are the preferred choice of researchers worldwide.
It’s all about high resolution, high color fidelity and optimized contrasting leading to insightful decisions.
Please contact us for a demo request or personal expert advice on our microscopy solutions for zebrafish research.
The basis for correct decisions in Screening & Sorting
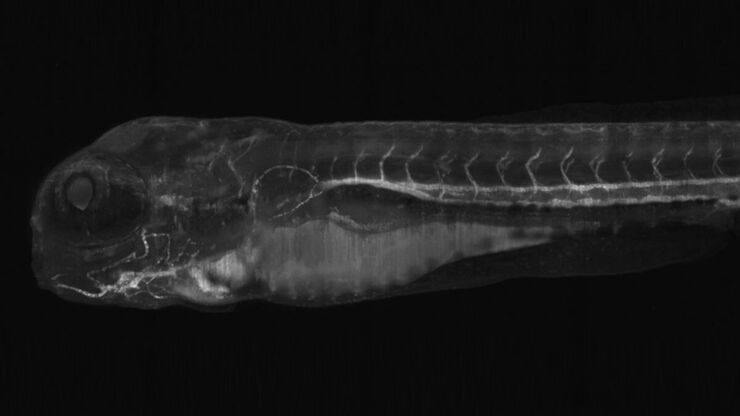
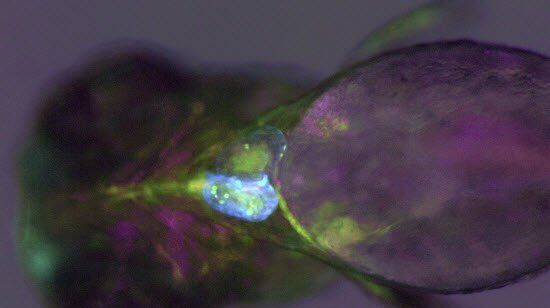
Do you struggle to see finest pigments and structural differences in your zebrafish? The identification of the right phenotype is crucial and can be demanding.
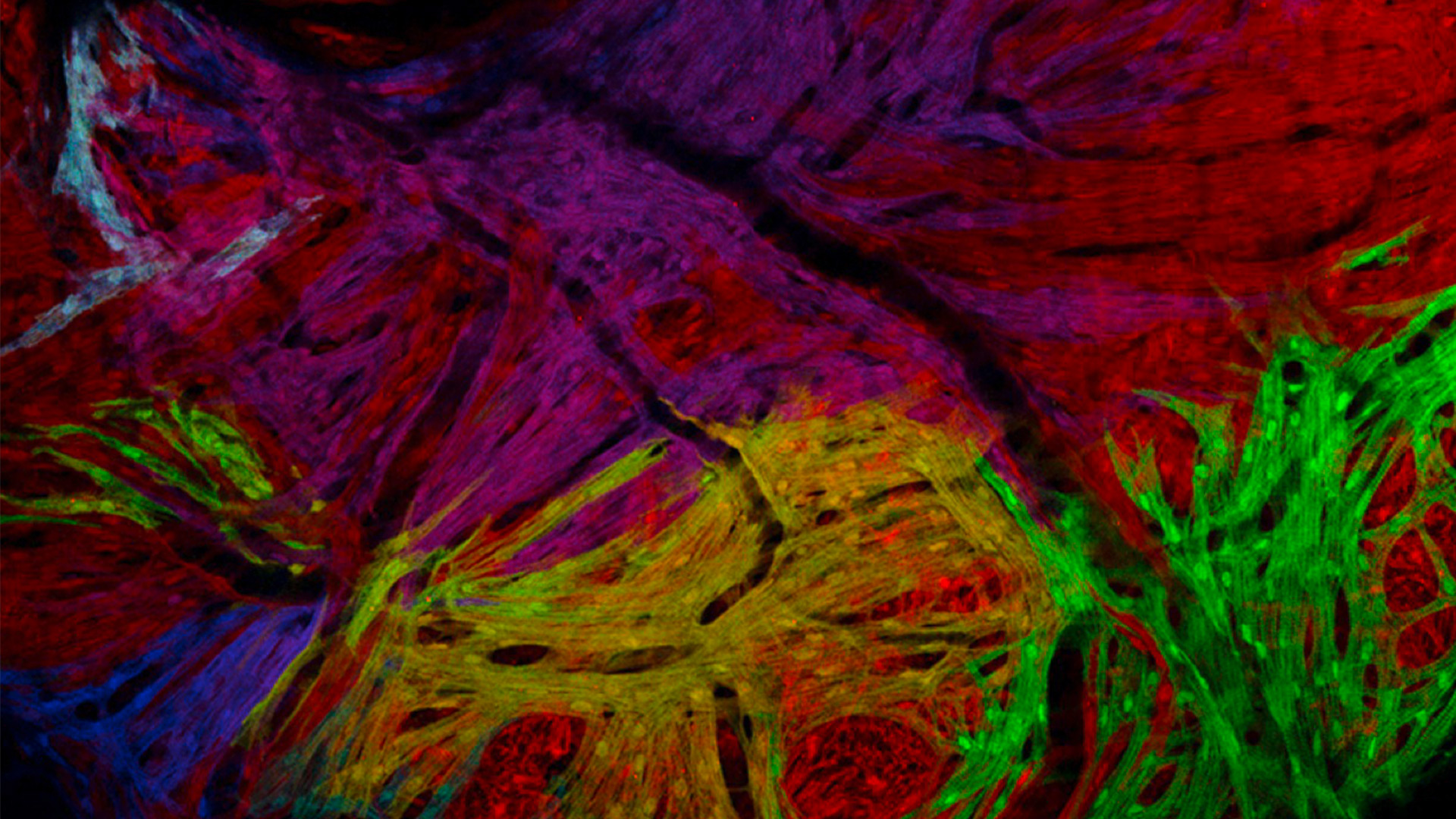
See more details at first glance – Leica zebrafish screening solutions allow you to recognize finest structures and more colors even at low magnification. This means clear understanding of the nervous system, heart, blood vessels, and pigmentation.
Improve your work experience by
- Easy selection of the perfect contrast
- Homogenous contrast over the entire FOV
- Steady contrast without readjusting over the entire zoom range
Make a difference in your screening & sorting with the Leica C.Elegans screening solution: M165 C and TL3000 Ergo.

Sample courtesy: Mosimann Lab, University of Zürich, Switzerland.
Fascinating information with different contrast options
Get the full color spectrum of your staining with the specially designed LEDs in the Leica TL bases.
- Evaluate staining and see realistic colors of your specimen in bright field
- Investigate internal structures with Rottermann Contrast
- Explore the smallest details with dark field illumination
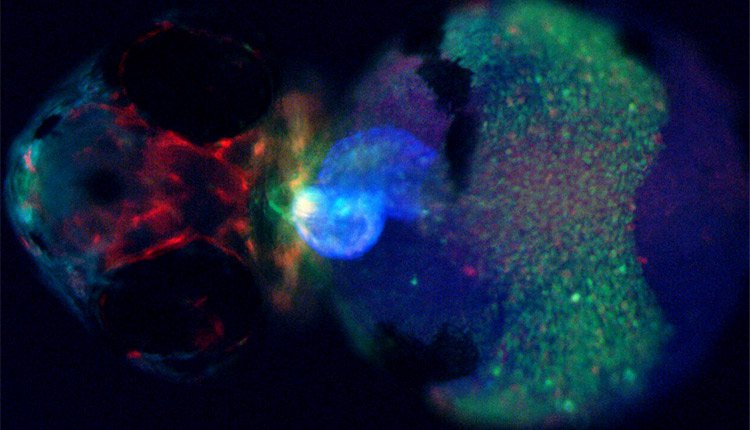
Fluorescence Screening: Don’t let background noise mask your signals
Detecting xFP fusion proteins can be very challenging. You try to keep the expression physiologically low for realistic protein levels. Signals are therefore faint and hard to identify.
In this situation your microscope solution should not be working against you with high levels of autofluorescence, e.g. from lens glue, glass, and the LED of your microscope base.
We solve these problems with
- A separate excitation channel in our fluorescence stereo microscopes, known as Triple Beam
- A specially designed mirror in our screening bases that doesn’t let autofluorescence come back
- Adjustable flaps covering the LED in the automated base to block autofluorescence during imaging
This results in a clear and strong fluorescence signal against a noise-free, black background.
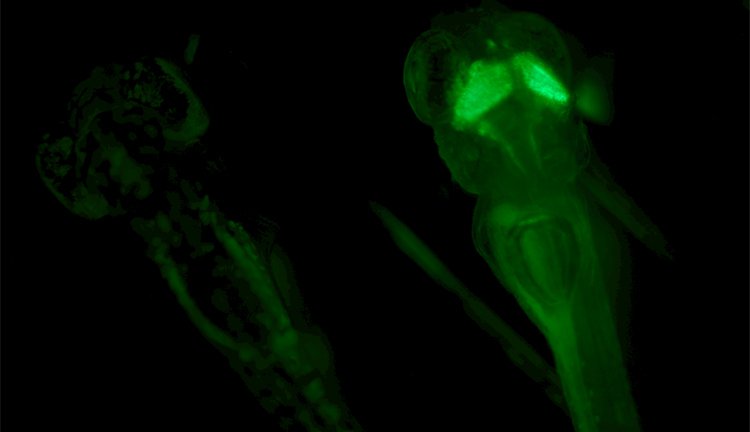
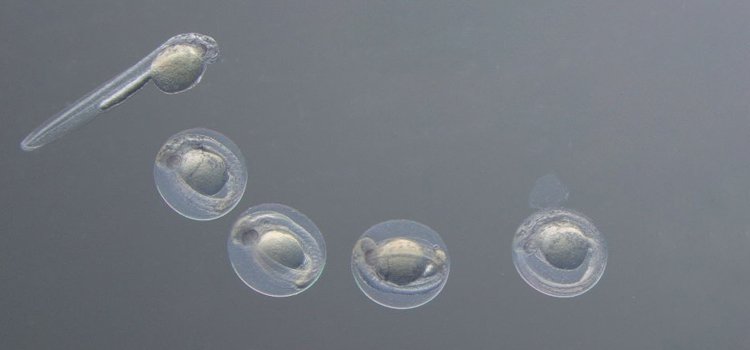
Manipulate your specimen with ease
Leica stereo microscopes allow easy orientation in 3D. This ensures that you see exactly where your tools are, so you don’t accidently injure your model organism.
Our zebrafish solutions enable you to
- Manipulate easily with a natural hand-eye-coordination
- Set injections and lesions precisely
- Position your sample ideally for imaging
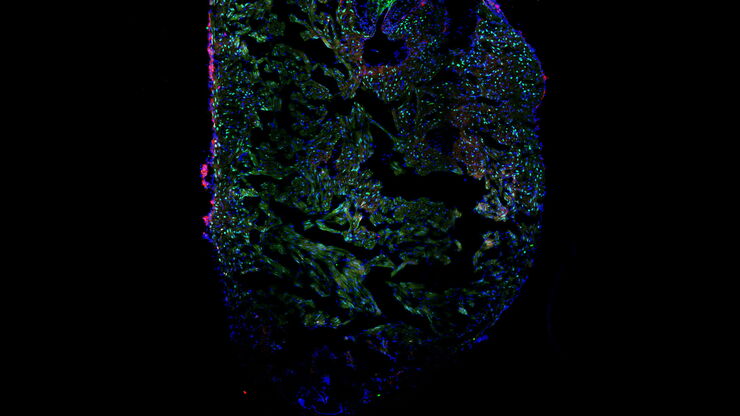
The perfect finish – publishable results
Functional imaging is one of the most demanding tasks in the laboratory. Leica supports you with fully automated stereo microscopes, like the M205 FA.
Work completely software based and control
- Fluorescence light, filters, and shutter
- Transmitted light contrast
- x/y/z-positions
- Camera
- Magnification
- Environment
With the fully automated TL5000 Ergo transmitted light base from Leica, functional imaging brings out the best of your fish. It provides highest NA of 0.9 and adjusts the aperture automatically to the zoom, in order to eliminate stray light.