Why use rodents and small animals as model organisms?
In order to better understand and develop treatments for diseases and health problems which effect humankind, i.e. cancer, heart disease, stroke, neurodegenerative disease, liver disease, arthritis, diabetes, obesity, etc., rodents and small animals are often used as model organisms for these studies. The rodents and small animals typically used in laboratory research are mice, rats, hamsters, guinea pigs, rabbits, hares, etc., There are significant anatomical, histological, hormonal, and genetic similarities between these rodents/small animals and humans, which justify their use as model organisms [2-3]. Additionally, there are practical reasons, for instance, the organs and glands of rodents and small animals are amenable to in vivo and in vitro manipulations and techniques used for disease, cancer, and stroke research and they have short life-cycles.
Are there guidelines and regulations for use of animals in laboratory experiments?
Research and experiments using laboratory animals is often regulated by local, regional, national, and international guidelines and laws for animal welfare and protection. An example of an international guidelines is the Directive 2010/63/EU [4] of the European Union (EU). There are also several international organizations which regularly publish updated guidelines and recommendations:
- Universities Federation for Animal Welfare (UFAW) [5];
- Federation of European Laboratory Animal Science Associations (FELASA) [6];
- Society of Laboratory Animal Science/Gesellschaft für Versuchstierkunde (GV-SOLAS) [7];
- American Society of Animal Science (ASAS) [8];
- Scientists Center for Animal Welfare (SCAW) [9];
- Asian Federation of Laboratory Animal Science Associations (AFLAS) [10].
Many countries also have guidelines and laws for animal research at the national, regional, and local level. Typically, these guidelines follow the principle of the 3R’s, as first reported by Russel & Burch in 1959 [11-12], i.e., encourage and favor methods in the laboratory which enable the following:
- Replacement: replace the use of animals in laboratory research;
- Reduction: the use of fewer animals while obtaining comparable or even greater amounts of valid data;
- Refinement: alleviate or minimize possible pain and suffering and promote the welfare of the animals.
Model organisms for research must be protected from certain infectious agents
It has been proven that in order to perform efficient and cost effective research and study with all laboratory animals, it is important that they are declared as “specific pathogen free” (SPF) [13]. The association FELASA has defined a list of pathogens which can have a significant influence on experimental results if the animals under study happen to be infected with them [14]. In fact, in many cases, the results tend to be irreproducible. Additionally, rodents and small animals usually do not recover from infection by these types of pathogens, so it is important to ensure that the enclosures where the animals are housed are free of these microorganisms. Whenever conducting experiments on animals which are not SPF certified, then there is a risk that any results obtained will have to be considered as non-reproducible and invalid. Such results will not be accepted for publication in international journals nor make any valuable contributions to improving the knowledge or state-of-the-art in the fields of laboratory animal science and human disease research.
In the case where a transgenic strain of a small animal, usually mouse or rat, should be transferred from one facility to another, the transferred animals must also be SPF. When mice and rats are transported in cages, there always the risk they will come into contact with wild mice or rats or their residues at some point during transportation, whether by train, truck, or plane. Such contact can result in a pathogen infection of the transported animals. To transfer a transgenic strain of mouse or rat into another facility without the risk of contamination, normally it can be done in 2 ways:
- fertilize a female transgenic mouse or rat, or even more simply its oocytes (immature egg cells), and then transfer the resulting embryos into the womb of a healthy mouse or rat in the target facility; or
- use the sperm from a male transgenic mouse or rat to perform in vitro fertilization ( IVF ) on a healthy female mouse or rat in the target facility.
Maintaining and using small laboratory animals
Small animal model organisms, such as mice, are expensive to maintain, so they cannot be held available in overly large numbers. Keeping the cages and facilities for these animals sanitized to maintain them as SPF is very labor intensive and expensive. In addition, as the guidelines and regulations mentioned above require every sacrificed laboratory animal to be reported, it is more efficient and cost-effective to sacrifice as few animals as possible. Therefore, the work done with each animal must be optimized to avoid waste and excessive cost.
Key factors for success in small animal surgery with microscopy
- Clear view of the animal’s targeted anatomical area is important → achieved by bright illumination in the microscope’s field of view from proper light sources and high transmission optics
- Sufficiently large area of the animal’s anatomy must be seen with the microscope → attained with a large field of view using eyepieces or camera (up to 23 mm)
- Plenty of space to work with surgical utensils under microscope is necessary → requires large working distance (up to 20 cm)
- No objects should obstruct your hands and utensils under the microscope and a comfortable head and sitting position while working are important to avoid strain on the neck, back, and arms → useful to have an ergonomic setup and accessories
- When working with animals in a fume hood or on a heating plate or pad, fine positioning of the microscope above the animal should be done easily with a sufficiently rigid stand that minimizes vibration
- Versatile and easy-to-set-up microscope configuration saves users time, reduce stress, and allow it to be used for multiple tasks
Small animal surgery used in stroke research
Stroke is one of the so called “lifestyle diseases” or “diseases of civilization“ [15]. The patient outcome after a stroke will depend on its severity and the availability of rapid treatment and medication. Recent research suggests that certain treatment methods can help stroke victims, such as cooling of patients, treatment with anti-apoptotic substances, among others [16-17]. These results are in part based on clinical research. The public interest in this type of disease research is quite high and, therefore, sufficient funding for it is often available. Rats and mice are definitely the most abundant mammalian model organisms used in laboratory research. They are not only small and relatively easy to maintain and care for in a lab setting, but there is also a wealth of knowledge and experience documented in many studies with these model organisms occurring over more than a century. Common guidelines and protocols ensure high reproducibility and allow subsequent comparison of results between stroke studies with different approaches.
Common approach for stroke investigations
First, usually an artificial stroke is produced in an animal, usually a middle cerebral artery occlusion (MCAO) [18-20]. Stroke studies can be performed with permanent occlusion (shutting or closing) or reperfusion (restoration of blood flow) of the cerebral artery or even both. Typically, one or more brain lesions result from the stroke. After the stroke, some animals receive a real and others a mock treatment, where the mock treatment results serve as a control study [21]. Recovery from the stroke is assessed by making, at certain intervals of time after its onset, appropriate experiments to determine changes in behavior and in the size of any brain lesions [18-19]. An example of a potential surgical setup with a Leica stereo microscope for stroke research is shown in figures 2 and 3 below.
Figures 2a and 2b are courtesy of M. Brown, M. Sixt, Sixt Group, Institute of Science and Technology, Klosterneuburg, Austria. Figure 3 is courtesy of K. Deligkaris, R. Brownstone, Sobell Dept. of Motor Neuroscience & Movement Disorders, Institute of Neurology, University College London, UK.
Challenges to overcome for work efficiency during stroke research
As mentioned above, guidelines for the use of animals in scientific experiments often follow the principle of the 3R’s or refine, reduce, and replace [11-12]. Also discussed above, every sacrificed animal has to be reported, researchers may have only a limited number of animals granted for their research. In addition, animal maintenance and experimentation are expensive, especially the cost for drugs and personnel, so efficiency when working with laboratory animals is important. Finally, reliable results from an efficient method are critical, as low reproducibility leads to skepticism.
Leica product range for small animal stroke surgery
The necessary tasks for rodent and small animal surgery concerning:
- Stroke
- Neurosurgery
- Research done with isolated organs
- Training and teaching of laboratory methods
are covered by a range of products offered by Leica Microsystems.
Using microscopes with high quality optics, practical and ergonomic stands, and versatile, integrated lighting solutions, users can work fast and efficiently. Some examples are shown above in figures 4 to 6 and described below:
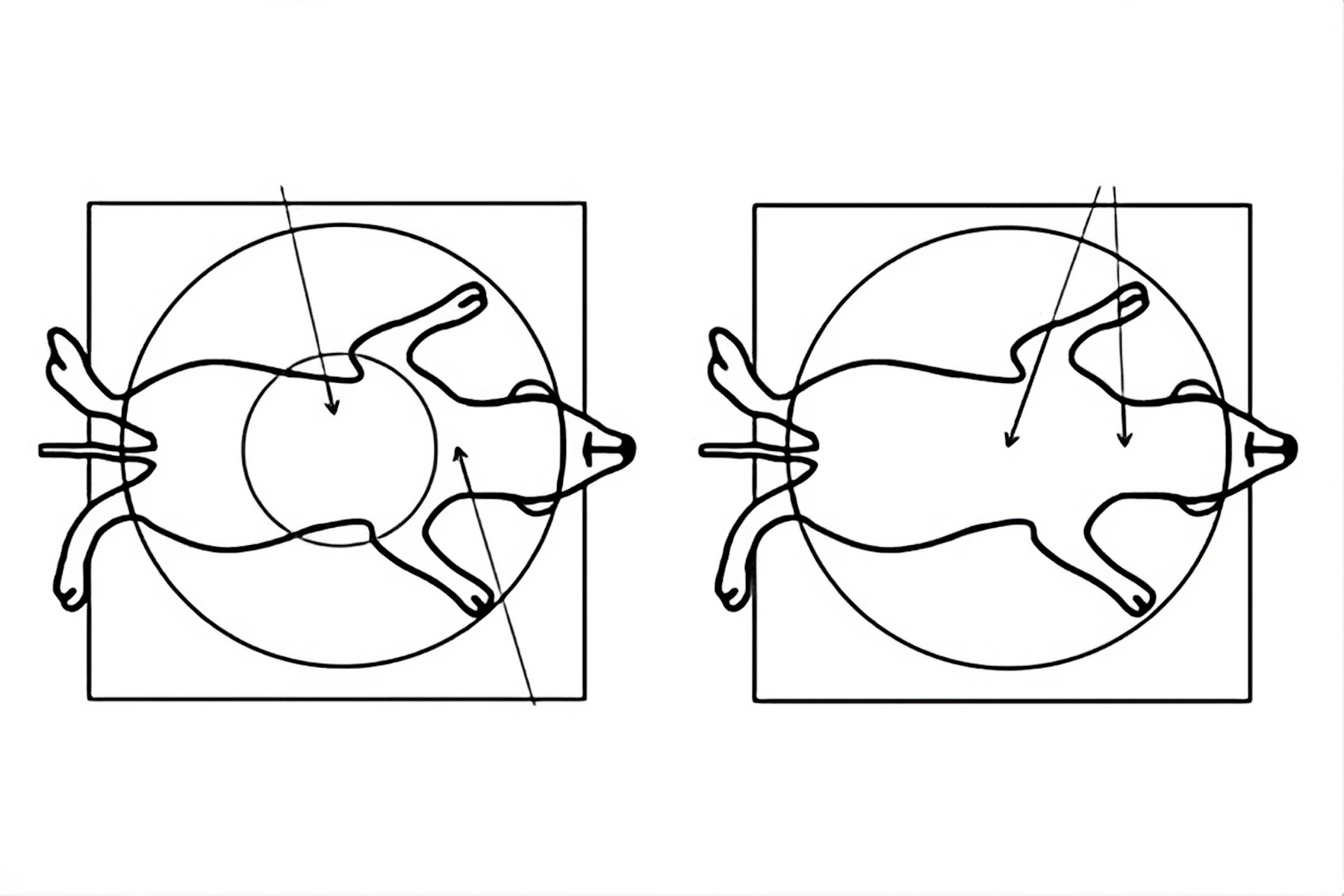
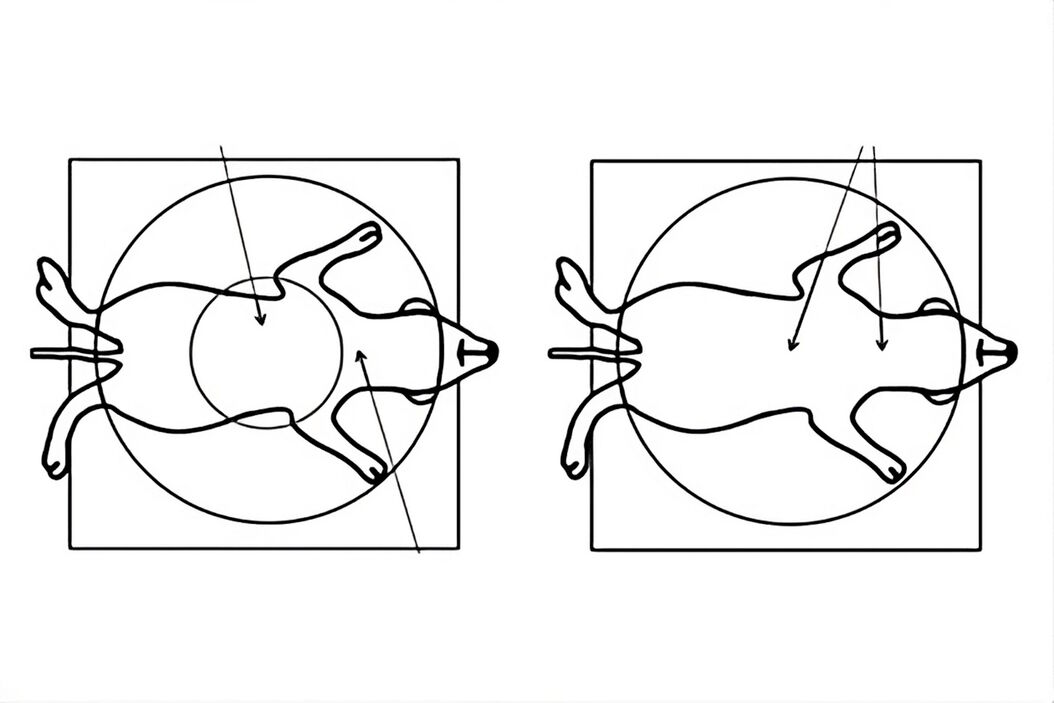
- An advantage for animal surgery would be a Greenough type stereo microscope (with 2 objective lenses, refer to figure 7) providing a larger overview of the anatomy with the ability to zoom in on a specific region, as well as an optimal balance between resolution and in-focus depth (FusionOptics [22]). A solution available at an attractive price is the A60 S and F [23] stereo microscope with ring light, LED3000 RL [24], and additional spotlight illuminators, LED3000 SLI [25].
- Common main objective (CMO) type stereo microscopes (with 1 shared objective lens, refer to figure 7) for surgery also offer a larger overview of the animal anatomy with the ability to zoom in on a specific region. They can be fitted with a variety of versatile illuminations. Examples are the M50, M60, or M80 [26] stereo microscope with the LED3000 NVI [27] or LED5000 NVI [28] near vertical illumination. Sometimes additional spotlight illuminators are also preferred. They can be mounted on a swingarm stand or a F12 I stand [29].
- For doing neurosurgery where live clamping on single nerves, e.g., sciatic nerve on the hindlimb, are necessary and resolution and working distance become essential, good solutions are the M125 [30] stereo microscope on a large swingarm stand with the LED5000 NVI [28] or LED5000 SLI [31] illumination.
- For scientists who want the same practical, flexible, and ergonomic setup as in a medical clinic, a good choice is the M320 T [32] microscope.
Advantages
The advantages for users are the following:
- Better visualization while working under the microscope leading to efficiency, reproducibility, and cost-effective procedures with less stress.
- Clean working conditions are required to keep the setup sterile. The least amount of cables lying around the setup is a key requirement when working in S1 or S2 laboratories or isotope areas. Stereo microscopes with integrated LED illumination means no cables nor cold light sources crowding the work space
- No need to adjust the lighting all the time - the light is where you need it with the LED5000 NVI
- To avoid a dark field of view while repositioning the specimen under the microscope objective, spotlights can also be connected to the stand
- If a less bulky illumination is needed, then use the LED3000 NVI which can be always directed onto the field of view, unlike spotlights
- Record procedures for method documentation and teaching by adding a Leica digital microscope camera [33]
Some Useful Hints:
- Working distance and lighting are critical for efficient use of the microscope
- Usual total magnification while working under the microscope is around 30-40x only
- Work even more comfortably and efficiently (ergonomically) with the Ergo modules
Small animal surgery used in cancer research
Over their entire lifetime, 1 out of 8 women can suffer from breast cancer. The risk of developing breast cancer (mammary carcinogenesis) increases with a woman's age and is related to her reproductive history. The mechanisms underlying the roles of hormones and other signaling proteins in mammary carcinogenesis are still poorly understood [34,35]. The hormone signaling pathway occurring in the breast (mammary gland) could provide explanations to the cancer promoting effects.
Common approach for onset of breast cancer studies
Mammary gland development occurs over multiple stages [34,35]. At birth, only a rudimentary milk duct tree is present. Hormones induce communication between the mammary epithelial and mesenchymal cells and regulate their development. During puberty, this regulation leads to mammary terminal end bud cells, eventually reaching the “fat pad” at which point they begin to form 2nd and 3rd degree branching.
Mammary epithelial tissue can be serially engrafted to cleared mammary fat pads for up to seven generations [34,35]. Transgenic mice expressing fluorescent proteins, such as enhanced green fluorescent protein (EGFP) or the red fluorescent protein DsRed, in the mammary gland tissue are often used for these type of development studies. At present, the surgical engraftment, i.e. serial transplantation, of mammary epithelial tissue is the sole way to assay comprehensively the ability of various types of cells to regenerate mammary tissue. This assay has been combined with different genetic mutant strains to define the relative contributions of hormone signaling and its downstream mediators to determine the regenerative potential of the mammary cells. The results have implications for mammary carcinogenesis.
It should be noted that all mice used for cancer research experiments in the laboratories of UPBRI, ISREC, EPFL are maintained and handled in accordance with Swiss guidelines for animal safety. All animal experiments are approved by the ethic veterinary committee of canton Vaud, Switzerland.
Refer to figures 8 to 11 below for images of a mouse transplantation surgery and tissue analysis laboratory at the UPBRI, ISREC, EPFL in Lausanne, Switzerland.
Challenges to overcome for work efficiency during cancer research
When fluorescent imaging surgically excised mammary tissue from a transgenic mouse line, the expression levels can be very low. This problem is especially true when fluorescent proteins like GFP are intentionally expressed close to endogenous levels. In practice, the lower concentrations of expressed GFP means that it’s often much harder to see. So for researchers working with GFP expressed at levels close to those of native proteins, fluorescence detection sensitivity can make the difference between finding and missing areas of the tissue having a fluorescence signal.
It is often necessary when fluorescently characterizing mammary tissue samples obtained over multiple generations of mice to compare them with the same lighting and microscope optics settings. So storing these values for easy recall and reproducibility is very important to save time. Understanding the fluorescence patterns of mammary tissue can require switching between the fluorescence and transmitted lighting of the base. Programming of the microscope controller, such as the SmartTouch or foot petal, and working with an encoded microscope with transmitted light base, such as the M165 FC or M205 FA fluorescence microscope with the TL5000 Ergo light base, can make this task much simpler to do.
Leica product range for small animal cancer research
Fluorescence characterization of surgically excised tissue from small animals, such as mice, can be done with the M125 [30] stereo microscope with fluorescence module (EL6000 [36] external fluorescence source). Other options are the MZ10F [37], or M165 FC [38] microscope (mid-range) with a TL3000 [39] transmitted light base and the M205 FA [40] microscope (high end) with the TL5000 Ergo [39] light base. Refer to figure 12 below.
Advantages
For the MZ 10 F stereo fluorescence microscope:
- Excellent resolving power (max 1.33 µm) with high numerical aperture (max NA = 0.25)
- Rapid magnification change with a zoom optics magnification range of 10:1
- Intense fluorescence illumination and highest fluorescence signal-to-noise (S/N) ratio with TripleBeam technology
- High quality images with plan achromatically or plan apochromatically corrected objectives [41]
- Rapid 4-position filter changing system (FLUOIII)
- User protection against UV radiation exposure
For the M205 FA and M165 FC stereo fluorescence microscopes:
- Go from overview to fine details with a zoom optics magnification range of 16.5:1 or 20.5:1
- Highest fluorescence signal to noise (S/N) ratio with TripleBeam technology
- Resolution down to 1.10 µm (max NA 0.3) with the M165 FC microscope and 0.96 µm (max NA 0.35) with the M205 FA microscope
- Achieve the highest resolution and depth of field currently possible for a 3D image viewed with a stereo microscope FusionOptics [22] available with the M205 FA microscope
- High-quality images with plan achromatic or plan apochromatic objectives [41]
- Reproducible results obtained easily due to instrument encoding
- Distortion-free observation of immersed or embedded specimens with the PLAN APO 2.0x CORR objective which allows elimination of refractive index mismatch
- Comfort and ease-of-use when doing complex experiments with the automated M205 FA microscope
- Rapid 4-position filter changing system (FLUOIII)
Summary
This report informs scientists and technicians working with small animals, such as mice, rats, and hamsters, for developmental biology or medical research, e.g., stroke or breast cancer, of different possible microscope setups for surgery. A key objective of the report is to help improve the routine work steps of animal surgery in terms of efficiency and cost-effectiveness. Because the demands of each laboratory can vary, a range of configurations and instruments are available to address specific tasks in the work steps or even enable more of them to be performed by one instrument. Useful information for work steps based upon the experiences of different labs are presented. The report can serve as a very useful reference or guidelines when setting up a surgical facility for studies in developmental biology or medical research labs where small animals, like mice, are used.
References
- K.R. Pritchett-Corning, G.B. Mulder, Y.Luo, W.J. White, Principles of Rodent Surgery for the New Surgeon, JoVE/J. Vis. Exp. (2011) iss. 47, Biology 2586, DOI: 10.3791/2586
- Hau, J., Schapiro, S.J., Handbook of Laboratory Animal Science, Volume II, Third Edition: Animal Models (CRC Press, 2011), ISBN: 978-1-4200-8458-0
- Fox, M.A., The Case for Animal Experimention: An Evolutionary and Ethical Perspective (University of California Press, 1986), ISBN: 0-520-05501-2
- Animals in science: EU actions for the protection of animals used for scientific purposes, European Commission, the European Union (EU).
- Universities Federation for Animal Welfare (UFAW)
- Federation of European Laboratory Animal Science Associations (FELASA)
- Gesellschaft für Versuchstierkunde/Society of Laboratory Animal Science (GV-SOLAS)
- American Society of Animal Science (ASAS)
- Scientists Center for Animal Welfare (SCAW)
- Asian Federation of Laboratory Animal Science Associations (AFLAS)
- Russell, W.M.S., Burch, R.L., The Principles of Humane Experimental Technique, (Methuen, London, 1959), ISBN: 0900767782
- The 3Rs: What are the 3Rs?, Definitions, Our impacts, The National Center for the Replacement, Refinement, & Reduction of Animals in Research (NC3Rs)
- R.K. Sellon, S. Tonkonogy, M. Schultz, L.A. Dieleman, W. Grenther, E. Balish, D.M. Rennick, R. Balfour Sartor, Resident Enteric Bacteria Are Necessary for Development of Spontaneous Colitis and Immune System Activation in Interleukin-10-Deficient Mice, Infect. Immun. (1998) vol. 66, no. 11, pp. 5224-5231
- M. Mähler, M. Berard, R. Feinstein, A. Gallagher, B. Illgen-Wilcke, K. Pritchett-Corning, M. Raspa, FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units, Laboratory Animals (2014) vol. 48, no. 3, pp. 178–192, DOI: 10.1177/0023677213516312
- M. Sharma, P.K. Majumdar, Occupational lifestyle diseases: An emerging issue, Indian J. Occup. Environ. Med. (2009), vol. 13, no. 3, pp. 109–112, DOI: 10.4103/0019-5278.58912
- H.M. Den Hertog, H.B. van der Worp, M.-C. Tseng, D.W.J. Dippel, Cooling therapy for acute stroke, Cochrane Database of Systematic Reviews (2009), iss. 1, art. no. CD001247, DOI: 10.1002/14651858.CD001247.pub2
- M.P. Mattson, C. Culmsee, Z.F. Yu, Apoptotic and antiapoptotic mechanisms in stroke, Cell and Tissue Research (2000) vol. 301, no. 1, pp. 173–187, DOI: 10.1007/s004419900154
- O. Engel, S. Kolodziej, U. Dirnagl, V. Prinz, Modeling Stroke in Mice - Middle Cerebral Artery Occlusion with the Filament Model, JoVE/J. Vis. Exp. (2011) iss. 47, Medicine 2423, DOI: 10.3791/2423
- K.L Schaar, M.M. Brenneman. S.I. Savitz, Functional assessments in the rodent stroke model, Experimental & Translational Stroke Medicine (2010) vol. 2, no. 13, DOI: 10.1186/2040-7378-2-13
- T. Chiang, R.O. Messing, W.H. Chou, Mouse Model of Middle Cerebral Artery Occlusion, JoVE/J. Vis. Exp. (2011) iss. 48, e2761, DOI: 10.3791/2761.
- M.Y. Cheng, E.H. Wang, W.J. Woodson, S. Wang, G. Sun, A.G. Lee, A. Arac, L.E. Fenno, K. Deisseroth, G.K. Steinberg, Optogenetic neuronal stimulation promotes functional recovery after stroke, Proc. Natl. Acad. Sci. USA (2014) vol. 111, iss. 35, pp. 12913-12918, DOI: 10.1073/pnas.1404109111
- D. Goeggel, A. Schué, D. Kiper, C. Müller, What is the FusionOptics Technology? Leica stereo microscopes with FusionOptics combine high resolution and depth of field for ideal 3D perception of samples, Science Lab (2023) Leica Microsystems.
- A60 Microscope Product Page, Leica Microsystems.
- LED3000 RL Illumination Product Page, Leica Microsystems.
- LED3000 SLI Illumination Product Page, Leica Microsystems.
- M50, M60, M80 Microscope Product Page, Leica Microsystems.
- LED3000 NVI Illumination Product Page, Leica Microsystems.
- LED5000 NVI Illumination Product Page, Leica Microsystems.
- F12 I Stand Product Page, Leica Microsystems.
- M125 Microscope Product Page, Leica Microsystems.
- LED5000 SLI Illumination Product Page, Leica Microsystems.
- M320 T Microscope Product Page, Leica Microsystems.
- Microscope cameras product page, Leica Microsystems.
- C. Brisken, V. Scabia, 90 Years of Progesterone: Progesterone receptor signaling in the normal breast and its implications for cancer, J. Molecular Endocrinology (2020) vol. 65, iss. 1, pp. T81–T94, DOI: 10.1530/JME-20-0091.
- C. Brisken, Progesterone signalling in breast cancer: a neglected hormone coming into the limelight, Nature Reviews Cancer (2013) vol. 13, pp. 385-396, DOI: 10.1038/nrc3518.
- EL 6000 Fluorescence Illumination Product Page, Leica Microsystems.
- MZ10 F Microscope Product Page, Leica Microsystems.
- M165 FC Microscope Product Page, Leica Microsystems.
- TL3000 & TL5000 Ergo transmitted light bases product page.
- M205 FCA & FA Microscope Product Page, Leica Microsystems.
- Objective Classes, Product Page, Leica Microsystems.





![Comparison of a Greenough (A, C) versus CMO (common main objective) [B, D] type stereo microscope used for small animal surgery. Figure 7: Comparison of a Greenough (A, C) versus CMO (common main objective) [B, D] type stereo microscope used for small animal surgery. The schematics in A and B show ray tracing from a side view of the microscope. Those in C and D show a top view as seen through the microscope. Due to the design of a Greenough optics there are 2 tilted focal planes, so parts of the rodent anatomy away from the viewing center cannot be in sharp focus simultaneously for both eyes. For the CMO optics there is 1 focal plane over the entire field of view and sharp focus for both eyes.](/fileadmin/_processed_/c/9/csm_rodent_surgery_cmo_vs_greenough_7202834a15.jpg)








