How are stem cells connected with the aging process?
Molecular medicine in gerontology aims to decipher the mechanisms of the aging process and develop ways of improving the quality of life in old age. Stem cell research is one of the most significant activities in this area.
Stem cells, of which there are various types, are vitally important in the aging process. Some stem cells are relevant for embryonic development. They are pluripotent, being able to form all cell types of an organism. These embryonic stem cells no longer occur in adults. They only continue to exist as somatic stem cells that are organ-specific, i.e. able to form two or three different cell types from only one organ type. Bone marrow stem cells, for instance, only differentiate as cells from the hematopoietic system like immune cells and red blood cells. Skin stem cells form new skin cells.
For us, adult stem cells are particularly interesting as they are already present in tissue and continue to exist during the aging process. However, organ-specific stem cells are no different from other cells in that the capacity of the tissue to regenerate declines rapidly in old age. We want to find out the reason for this and look for ways of stimulating adult stem cells to make them divide and regenerate the tissue more effectively. I, for example, have studied the signal proteins p53 and p21.
What are the functions of the signal proteins p53 and p21 in the aging process?
I need to give a bit of background information here. There are several reasons why an organism ages. An important aspect in this process are the telomeres at the end of the chromosomes that serve as a kind of "protective cap" for preserving the genetic information of a cell. Because of the so-called "end-replication problem", a small piece of this protective cap is lost every time the cell divides. From a certain point, the telomeres are no longer able to perform their protective function. This can lead to the loss of genetic information, chromosomal instability and thus to a degeneration of the cell. Cells are often the cause of tumor development. Therefore, a mechanism is triggered that stops such cells from dividing further. This protective mechanism that is supposed to prevent tumors from developing is activated and controlled by an extremely important signal molecule – protein p53. The importance of this protein can be seen by the fact that p53 has stopped functioning properly or is completely missing in 90 per cent of all cancer cases.
This shortening of the telomeres happens in stem cells as well. Here too, p53 is activated to stop further division of the stem cells.
If you imagine a cascade of signals, protein p21 is beneath p53 and is dependent on its activation. If p53 is activated, it can initiate two different signal paths that decide the fate of the cells. On the one hand, activation of p53 can lead via p21 to the situation that the cell is still able to function but no longer able to divide. The cell’s division capability is permanently suppressed: we speak of senescence here. On the other hand, when the shortening of the telomeres is too far advanced and there is further damage to the genetic information – for instance as a result of UV radiation – p53 can initiate apoptosis via the PUMA protein. In this case there is a targeted cell death.
What is the current situation in stem cell research, what approaches are being pursued?
We are now increasingly able to comprehend what happens due to the shortening of the telomeres in the cell caused by aging and the resulting loss in functioning and regeneration potential of the stem cells. Our ideal aim is to slow down the aging process and to improve the regeneration potential of the aging organism as well as to prevent increased tumor development. To do this, we need to break down these processes in detail and understand the function of stem cells in the aging process. This knowledge should help us find out how to steer the various signal proteins "intelligently" to our benefit.
Stem cells are an important area of research. In Germany, scientists are concentrating more and more on adult stem cells due to the ethic qualms about using embryonic stem cells. However, the issue of ethics is not the only problem with embryonic stem cells. Because of their capability to differentiate into all types of tissue and cell, they theoretically offer huge application potential. In reality, however, it is difficult in clinical applications to get the embryonic cells to the right place, to make them produce only the desired cell type and not cause any unwanted degenerations.
It could therefore be an advantage for scientists to use their own organ-specific stem cells. Firstly, these adult stem cells are already at the place where they are needed, and secondly, they can only differentiate into a few organ-specific cell types. This reduces the probability of cell degeneration.
In hospitals, this possibility is already being used by transplanting patients’ own or foreign hematopoietic stem cells. Chemotherapy and radiation used to treat cancer also kill off other frequently dividing cells such as hair cells and those of the immune and hematopoietic system. The stem cells are taken from the patient before treatment and given back afterwards to restore the function of the immune and hematopoietic system.
This is also how, for example, leukemia is cured, by doctors transplanting bone marrow stem cells from a healthy donor. This method of stem cell transplantation is intrinsically quite easy, as the cells can be distributed via the bloodstream. However, it is not as easy to do in solid organs such as the liver. It would be a great advantage here to be able to use stem cells of the organ itself.
Telomeres are not the only reason for aging. It is also caused by substances naturally produced in the body such as soluble factors, cytokines and hormones circulating in the blood. As we grow older, there are changes in the way they are released and in the ability of our body to react. This leads to a deterioration of the function of the cells or whole organs. This is another area that needs researching in more detail. As such endogenous substances are detectable in our blood, they have the potential to serve as biomarkers, giving us information on the "age" of an organ or the whole organism.
What insights did you gain from working on your dissertation?
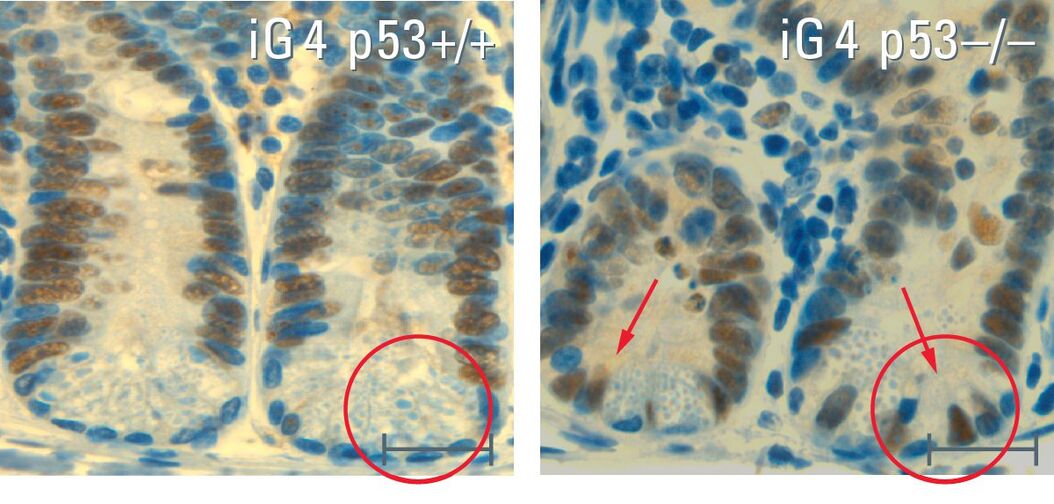
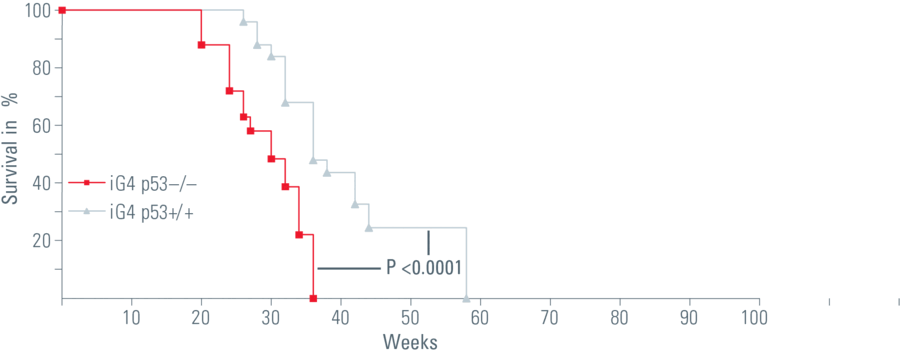
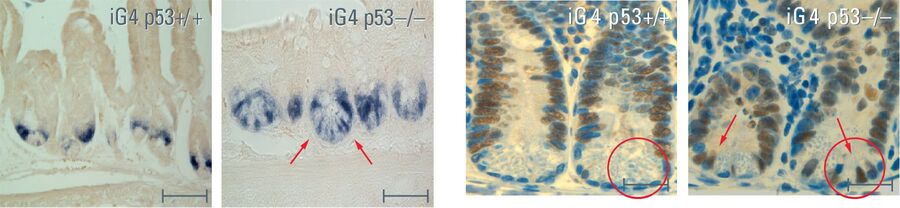
In my dissertation I used a mouse model that simulated the human aging process. In this system, the signal protein p53, that activates the protective mechanism as a response to short telomeres, was switched off in the intestinal epithelium. The loss of p53 caused the mice to age more quickly. Non-functional stem cells with genetic alterations remained, the mice died sooner.
So the mice had a shorter lifespan but no intestinal tumors. In fact, we found that this was due to the loss of intestinal stem cells, which are necessary for regular regeneration of the intestinal epithelium. We also discovered that the few remaining stem cells that try to preserve the function of the intestinal epithelium often exhibit genomic mutation, which leads to malfunctioning of the organ.
Why is the intestine the focus of your studies, that is to say, why did you choose it for your research?
We had several reasons for choosing the intestine as a model system:
In the aging model of telomerase-deficient mice we used, the hematopoietic system and the intestines show the greatest effects. The diminished regeneration capability of the intestine leads to malfunctioning. The intestine is no longer able to absorb and utilize nutrients. We also know the location of the stem cells in the intestine and which cell types they differentiate into. Apart from this, the intestine has a good, easy-to-examine structure, i.e. a strongly pronounced phenotype. The stem cells divide frequently and the differentiation can be observed along the villi as far as their tips.
To study the role played by p53 in the aging process, we wanted to switch off protein p53 in these mice. As already mentioned, p53 is involved in the development of most cancer types. The absence of this protein in the entire murine organism leads to the development of lymphomas, which makes an examination of p53 in the aging process considerably more difficult. Because we wanted to avoid that, we used an aging model with telomerase-deficient mice in which the protein p53 is only switched off in the intestine.
Do you think that your findings will contribute to new research or even therapy approaches with regard to tissue aging or tumor development?
These insights are of crucial significance for the understanding of aging processes. The most important thing is what happens to the stem cells: When there is no more p53, the non-functional intestinal stem cells are no longer removed and lose their capacity to form an intact intestinal epithelium. The function of p53 is therefore a cell barrier – it sorts out non-functional stem cells and prevents both tumor development and the regeneration of an organ with non-functional differentiated cells. Interestingly, the activity of p53 decreases in the human system in general, which could partly account for the increase in tumor development and also for a more pronounced aging process. We are now looking for a way to preserve or increase the activity of p53. It would be helpful if we could lower the number of changed stem cells and thereby slow down the aging rate of the tissue. This, of course, would also prevent tumor development. On top of that it should also keep organs functioning better and for longer. Our ultimate goal is not to extend life span as such but to improve the quality of life in old age. Many diseases such as dementia, liver cirrhosis and pain in the joints are age-related. It would naturally be good if we could boost the regeneration capacity of our own organs so that we could grow old healthily with a high quality of life.
Why do you use laser microdissection?
Laser microdissection offers the great advantage of being able to isolate single cells or a particular cell type. A cell culture consists of a uniform population of a certain cell type. However, tissue is made up of different cell types. An organ therefore mainly consists of different differentiated somatic cells that maintain its particular function. The cells that are specific to this organ only account for an extremely small percentage of the entire organ. Looking at the organ as a whole, they are rather underrepresented. To study special stem cell-specific mechanisms, we can use laser microdissection to cut out stem cells visualized by specific markers in the tissue. This is the only way to examine both defined “stem cell pools” and differentiated cells in a targeted manner. We can now show the important signal cascades and proteins in the stem cell or another differentiated cell population and exactly how they are regulated. Laser microdissection has opened up totally new avenues here.
What features of the LMD system do you find especially useful?
The most useful features of the Leica LMD system are the precise isolation of cells with a laser and the use of gravity to transfer the dissectate to the cap. The system enables me to work hygienically and RNA- and DNAse-free. It ensures fast, gentle and contamination-free isolation of the sample while being beautifully easy to operate. The touch screen is particularly time-saving and convenient. With this tool we can directly label and dissect as many cells of a certain type as we want. We also like the cap control feature, which enables us to check that the dissectate is in the intended lid of the Eppendorf cup we use.
What exciting issues relating to stem cells and aging processes still need to be researched?
In future, the study of single, specific cell populations – as opposed to complete tissue – will gain more and more importance. Clean and contamination-free isolation of the cells is important here.
It should be mentioned that there are not only genomic changes, i.e. direct modifications of the DNA sequence, but also epigenetic changes. These do not directly involve loss of DNA, but lead to a wrong or different reading of the genetic information.
We want to examine and understand what turns a cell into a stem cell or differentiated cell even though the DNA itself is always the same. There is still a great need for research to throw light on these mechanisms: the proteins that are expressed in the process and how we could ultimately control a regeneration of the cells. Consider chronic liver diseases like cirrhosis, for example. At first, the liver is able to replace the dying liver cells. Gradually, the regeneration capacity of the differentiated liver cells is exhausted and more connective tissue is formed. These fibrotic areas occupy the space of the liver cells without being able to perform their function. In this scenario it would be an advantage to not only activate the liver stem cells, but also stimulate them into producing liver cells.
Of course, it’s important for us to find out which stimuli the stem cells need to differentiate into the relevant cell types. This would not only give us key information on aging processes. We could also use the knowledge to treat chronic diseases.

Downloads
Download Application Note "Comparative Genome Hybridisation on Single Intestinal Crypts"