What kind of specimen do you use?
One of the first things to consider when selecting a research microscope is the type of specimen you want to explore. For fixed samples on glass slides, you can use an upright microscope. Living cells demand special microscope functions, because they are kept in large cell culture vessels filled with media.
An inverted configuration, with the objective below and the condenser above the specimen, allows the objective to be close to the cells, but at the same time good accessibility to them, e.g., for micromanipulators.
In addition, living cells require an adequate environment to survive. Temperature and CO2 concentration have to be kept at certain values, so a climate chamber is necessary.
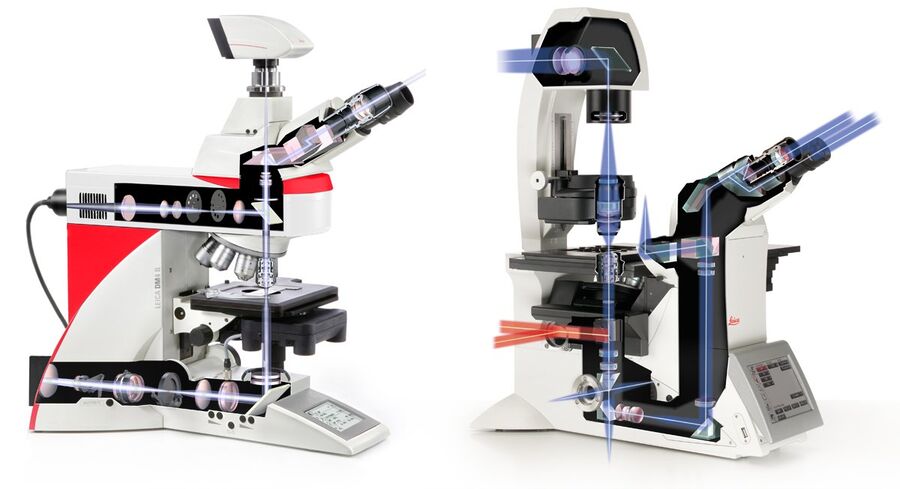
Left) An upright microscope features the objective above and the condenser below the specimen on the stage. Right) With an inverted microscope, this setup is turned upside down, giving the users more space from above to access the specimen, while below the objective is within the required proximity of the specimen for focused imaging.
Related articles on specimen types
A Guide to Neuroscience Research
A Guide to Using Microscopy for Drosophila (Fruit Fly) Research
A Guide to Zebrafish Research
A Guide to Model Organisms in Research
A Practical Guide to Virology Research
Guide to Microscopy in Cancer Research
A Guide to Super-Resolution
In which dimensions do you work?
Specimens have three dimensions: length (x), width (y), and height (z). Some, such as histological sections, are imaged only in the xy direction. Other applications, like with living cells, demand acquisition in the z dimension. To image specimens in 3D, a motorized objective revolver is recommended to guide stepwise through the focus range. The imaging software should superpose together the single plane images for 3D visualization.
For living cells, time can be important as well. In that case, the system stability is critical. Because temperature changes influence the imaging system, effective counter measures are essential. Automatic focus adjustment, as with the adaptive focus control (AFC), counteracts these thermal influences and maintains the predefined focus.
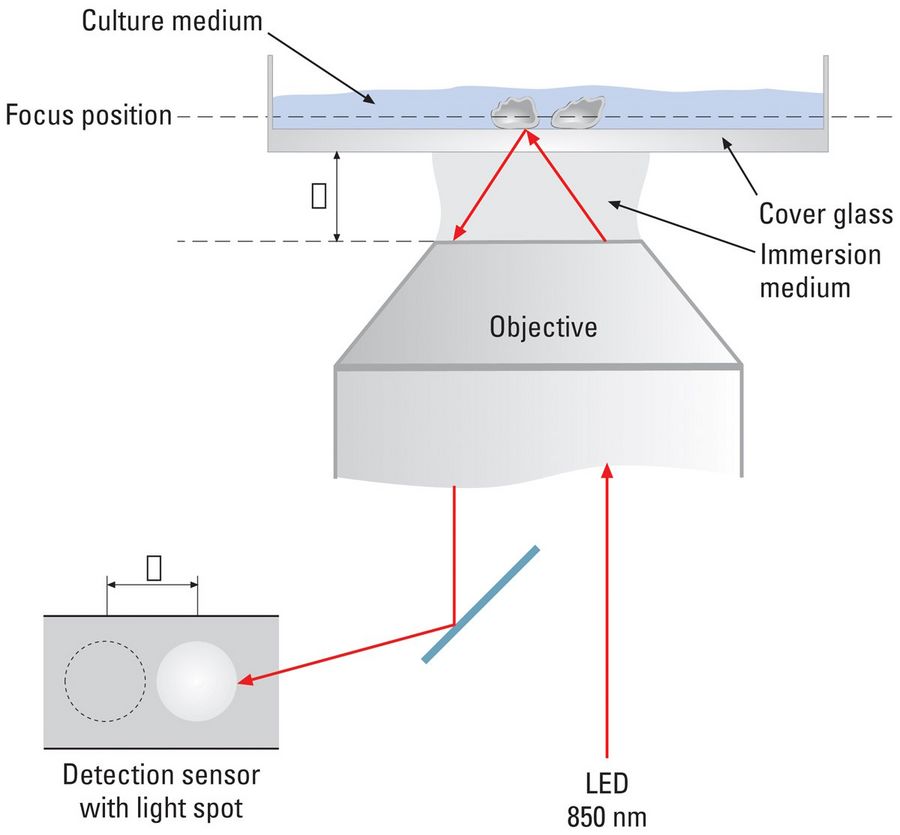
The adaptive focus control (AFC) automatically stabilizes the microscope focus during long-term time-lapse acquisition. A sensor detects movements of an LED light beam (850 nm wavelength) on the cover glass of the specimen. The cover glass position can change due to thermal activity and the resulting temperature changes.
Related articles on Spatial Biology
A Guide to Spatial Biology
Empowering Spatial Biology with Open Multiplexing and Cell DIVE
Which contrast methods are best?
The majority of cells don’t have enough intrinsic contrast, making it difficult to see fine details with a microscope. Contrast methods, like phase contrast and differential interference contrast (DIC), can fix this problem. Phase contrast needs special objectives and DIC certain prisms.
Specimens can also be stained with fluorescent dyes or express fluorescent proteins. Fluorescence microscopy needs special filter cubes so the correct light wavelengths excite the dyes or proteins.
What about the light source?
The contrast method determines the light source. Transmitted light illumination, either LED (light-emitting diode) or halogen, is typical for brightfield, phase contrast, or DIC microscopy. LED or mercury, xenon, or mercury metal halide lamps are used for fluorescence microscopy.
Related articles on contrast methods and fluorescence
Optical Contrast Methods
Phase Contrast and Microscopy
Differential Interference Contrast (DIC) Microscopy
Fluorescent Dyes
How to Prepare your Specimen for Immunofluorescence Microscopy
Fluorescent Proteins - From the Beginnings to the Nobel Prize
Fluorescence in Microscopy
Do you want to document or publish your results?
To acquire a specimen image, you need a digital microscope camera. In the case of fluorescence live-cell imaging, a sensitive camera is recommended to minimize excitation light. Recently, sCMOS cameras have become popular due to their high quantum efficiency and acquisition speed.
Furthermore, a large field of view (FOV) helps you find interesting areas more easily and quickly. Research microscopes now feature a 22 mm FOV at the camera port.
Often, it is not sufficient to just take an image of your specimen, but you often need to analyze the acquired data. Easy-to-use imaging and analysis software, especially now using artificial intelligence (AI), helps you obtain data and analyze it accurately.
Related articles to image analysis
Technical Terms for Digital Microscope Cameras and Image Analysis
Life Science Research: Which Microscope Camera is Right for You?
Get to Insights Faster and Easier with AI Image Analysis Tools
How Efficient is your 3D Organoid Imaging and Analysis Workflow?
Do you need 3D information from thick specimens?
Thick samples are a challenge for widefield microscopy where the whole specimen is illuminated. Identifying in-focus features can be difficult due to scattered light.
Computational Clearing (CC) helps you obtain images free of out-of-focus light, so interesting structures are distinguished easily. Instant CC (ICC) is applied to a single image plane and Small or Large Volume CC (SVCC or LVCC) is combined with deconvolution which reassigns photons to the place of origin.
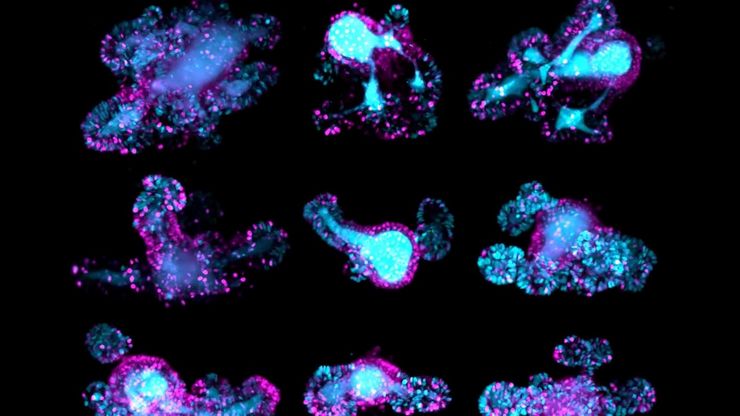
Light-sheet imaging (LSI) is useful for thick, delicate, or dynamic specimens, e.g., embryos, spheroids, or organoids. Other methods typically expose specimens to higher light doses, limiting observation time. The non-invasive nature and 3D acquisition of LSI make it suitable for long imaging times.
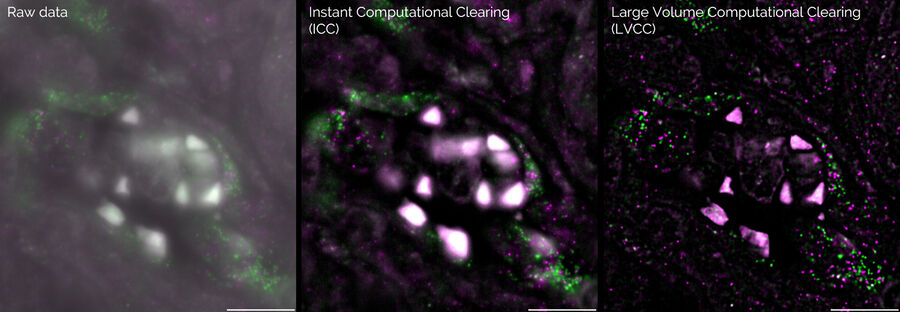
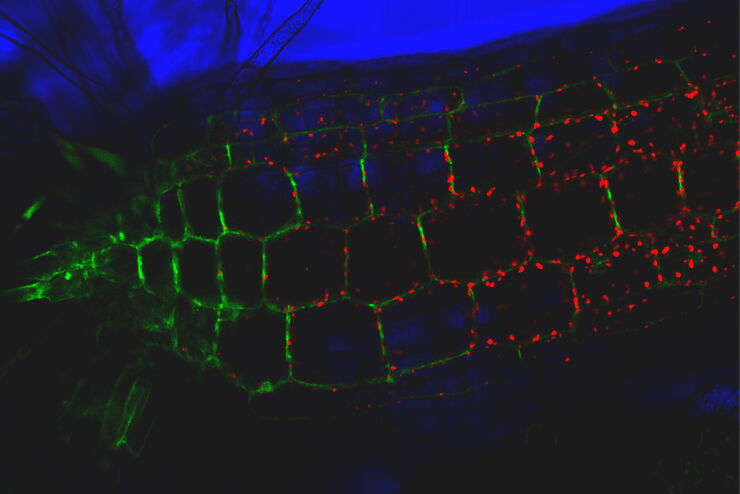
Single molecule RNA-FISH in cancerous tissue labelled with RNA-01 (green) and RNA-02 (magenta).
Left) Raw data, Middle) With ICC (instant computational clearing), and Right) After LVCC (large volume computational clearing). Courtesy of A. Moor, University of Zurich, Switzerland.
Related articles to image analysis
Real Time Images of 3D Specimens with Sharp Contrast Free of Haze
Going Beyond Deconvolution
Get to Insights Faster and Easier with AI Image Analysis Tools
How Efficient is your 3D Organoid Imaging and Analysis Workflow?
Focus on Long-Term Imaging in 3D with Light Sheet Microscopy
Do you want to manipulate your cells on the microscope?
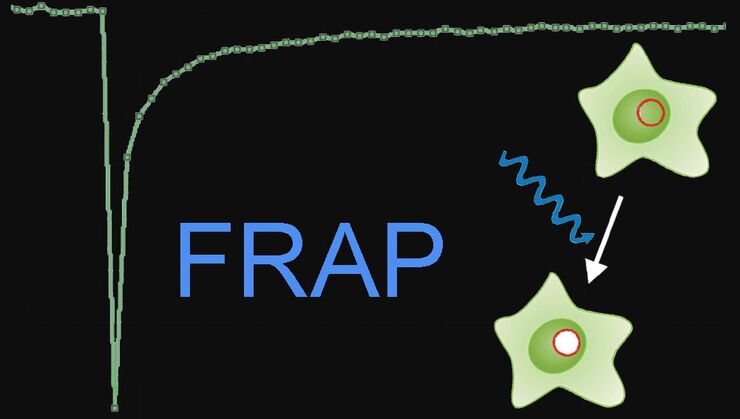
During the last few years, photo-manipulation of the specimen has become popular. So living cells are not only imaged, but manipulated with light. Fluorescence recovery after photobleaching (FRAP) is one example which helps untangle dynamic cellular processes. Förster resonance energy transfer (FRET) verifies interactions between cellular components. For these kinds of techniques, often additional light sources must be integrated into the microscope’s light path which is not trivial.
The Leica Infinity Port is a universal solution which couples additional light sources into the microscope’s light path to do FRAP, photo-switching, ablation, or optogenetics. There is no reduction in image quality. With the right adapter, even homebuilt devices can be added.
Related articles on cell manipulation
Step by Step Guide for FRAP Experiments
Fluorescence Recovery after Photobleaching (FRAP) and its Offspring
Live-Cell Imaging Techniques
What is your budget?
One important question is microscope cost. Predefined configurations, like the Mica Microhub, are useful for special applications, but a user-decided configuration may be cheaper.
If microscope requirements change with time, then an upgradable system has certain advantages, allowing you to change it for evolving needs.
A modular microscopy platform, e.g., the DMi8, enables you to start with an affordable system which can be upgraded later on with changing demands.
Who will use the microscope?
The demands of multiple users can vary dramatically. At university laboratories, user-skill levels can range from very experienced to beginners. Thus, an easy-to-use microscope system with an intuitive software, like the Leica Application Suite X (LAS X), helps users get started acquiring data quickly.
FAQs
The main types include widefield microscopes, i.e., compound or stereo, confocal, and multiphoton microscopes. Confocal offers the best resolution, then compound, then stereo. Stereo microscopes allow a very good 3D perception. Additionally, the Mica Microhub is an automated microscope which unites widefield and confocal imaging.
When imaging live cells, the microscope settings should minimize cell damage from light exposure, while maintaining a sufficient contrast, resolution, and signal-to-noise ratio (SNR). Leica microscopes which enable live-cell imaging are the DMi8, THUNDER Imager Live Cell & 3D Assay, Mica Microhub, Viventis Light Sheet, and STELLARIS confocal systems.
There are several types of Leica software available for analysis of life-science-specimen images. Examples include the Leica Application Suite X (LAS X) Core, LAS X Life Science, LAS X Navigator, LAS X Office, and Aivia AI software.