Investigating eye morphogenesis
The mouse lens can be studied as a model system to unravel the morphogenetic events, such as changes in cell shape as well as cell migration and proliferation, which are essential to the formation of the lens [1,2]. Disruptions of these processes can lead to ocular birth defects, but the origins of these disruptions are not well understood [1,2]. Scientists use a combination of mouse genetics, chicken embryology, and cell culture to create model systems which can help them elucidate the mechanisms of eye morphogenesis at the cellular and molecular level.
Challenges
Thick specimens are often challenging for widefield microscopy, due to the haze or out-of-focus blur produced by light scattering [3]. The haze can obscure structures of interest deep inside the specimen.
Methods
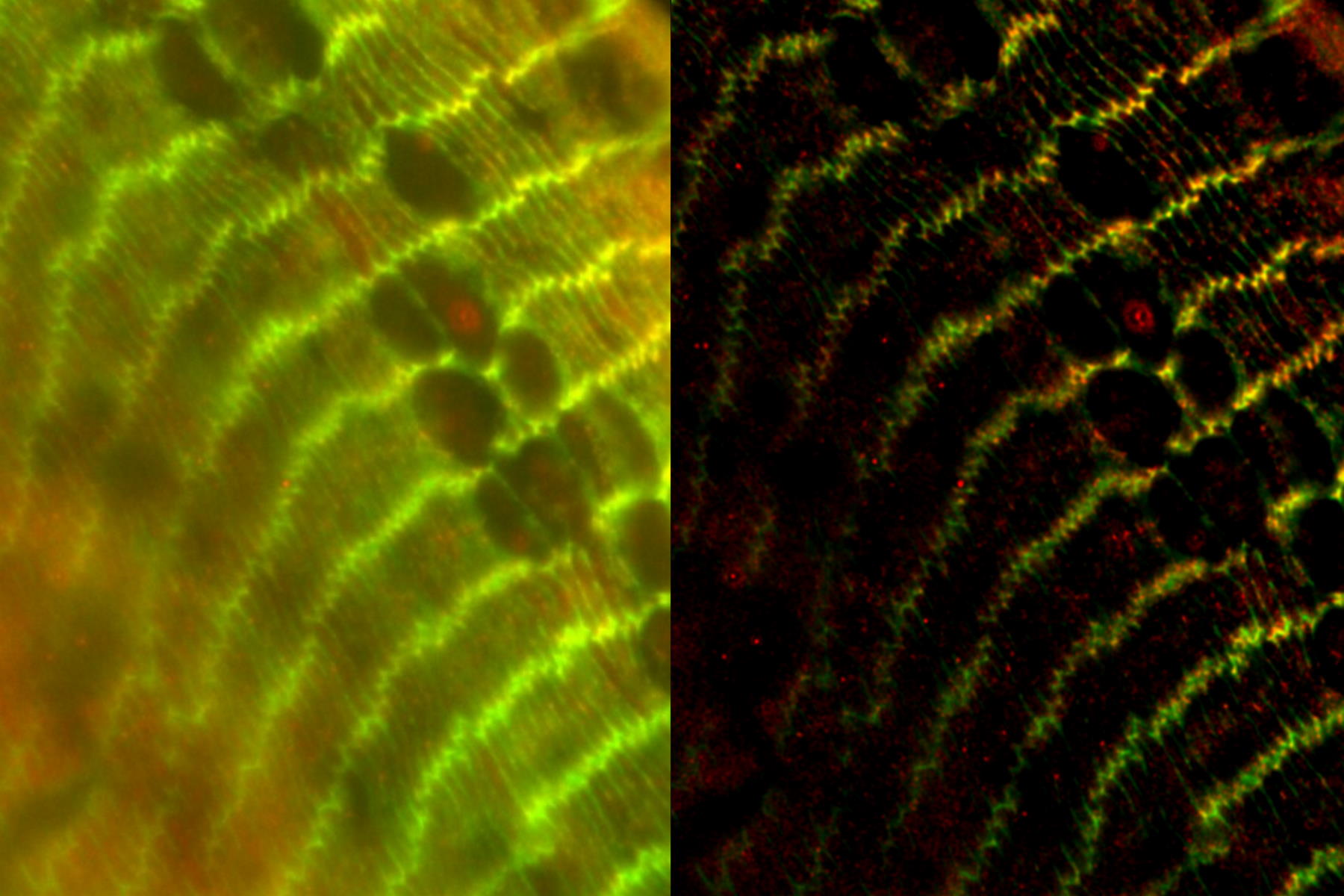
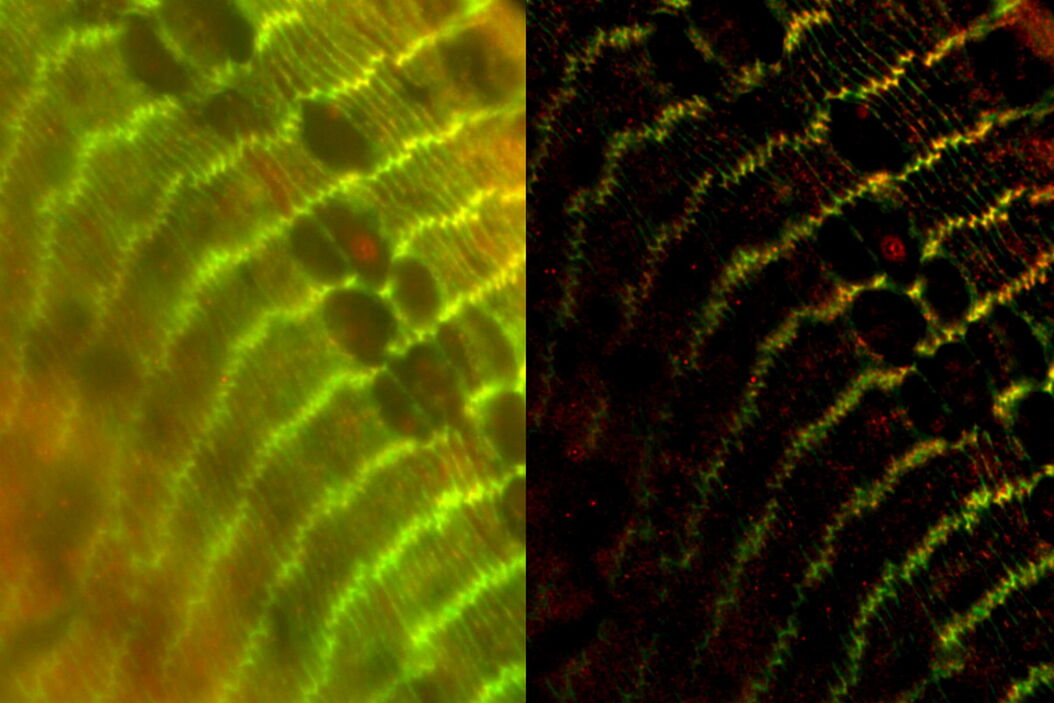
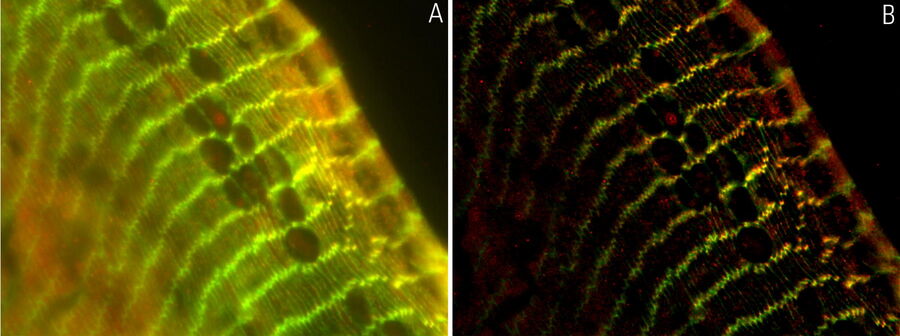
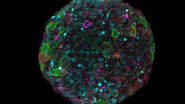
The specimen imaged is a thick transversal section of a mouse adult fiber lens. It was stained with anti-ß-catenin antibodies making BDB610153 membrane proteins appear green, anti-Afadin antibodies making PA1-25076 actin filament-binding proteins appear red, and Hoechst making the nuclei appear blue. The section was imaged with a THUNDER Imager 3D Assay and large volume computational clearing (LVCC) was applied [3,4].
Results
Images of the mouse lens section are shown below in figure 1.
Figure 1: A) raw widefield image and B) image after LVCC of a transversal mouse adult fiber lens section. The images show the cell organization where green indicates BDB610153 membrane proteins, red Afadin actin filament-binding proteins, and blue nuclei. Image courtesy of Dr. Nathalie Houssin, the Plagemen lab, Ohio State University, Columbus, USA [1].
Conclusions
The results presented here show that using a conventional widefield system to acquire images of the mouse fiber lens section gives very blurry results [3,4]. However, with a THUNDER Imager 3D Assay, the ability to visualize the regions of interest is greatly improved. It enables the user to obtain a clear view of details, even deep within an intact specimen, in real time without haze.