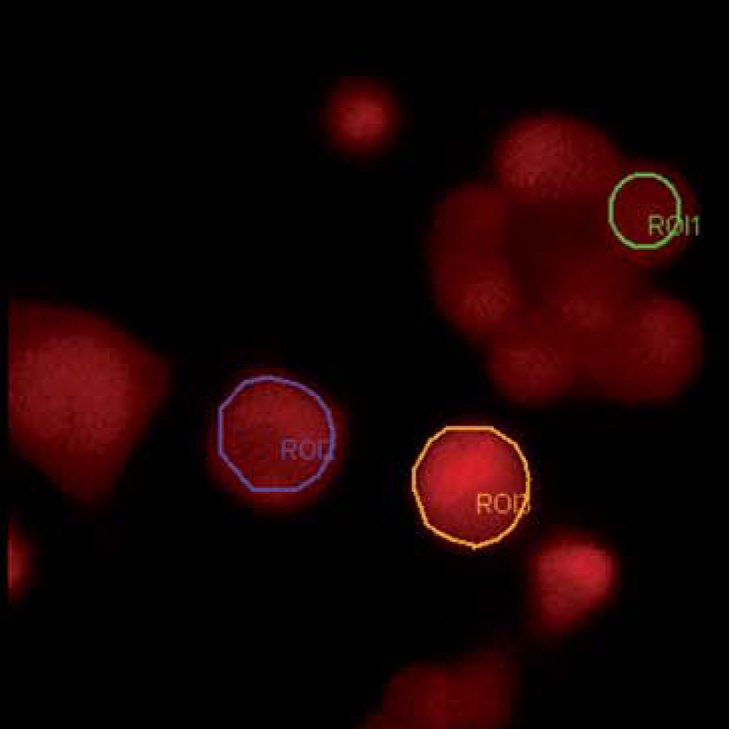
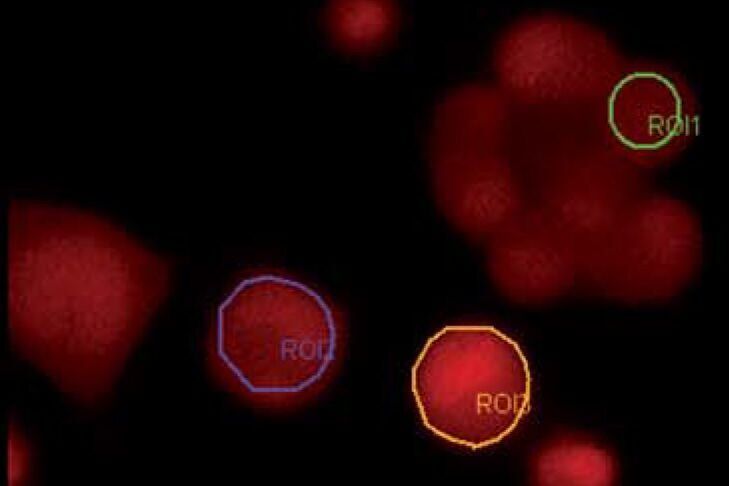
Ca2+ dyes
Ion sensitive dyes are fluorescent molecules which reversibly bind to specific ions. These dyes are sensitive to changes in ion concentration either by increase or decrease of fluorescence. A measure for the affinity of ion binding to the dye is the dissociation constant (Kd). The lower the Ca2+ binding affinity the higher the Kd (Ca2+). There are two major groups of ion-sensitive dyes: 1. Single wavelength dyes, like Fluo3 and Calcium Green which are suitable for non-ratiometric measurements. The intensity of the emitted fluorescence light increases proportional to free ion concentration. 2. Dual wavelength dyes are used for ratiometric measurements. These indicators can also be divided in two groups:
Dual emission ion indicators:
These dyes are excited at one wavelength and show a shift in their emission spectra upon binding specific ions. A well-known and often applied indicator of this group is Indo1.
Dual excitation ion indicators:
These dyes are excited at two different wavelengths The intensity of the emitted fluorescence light changes depending on the ion concentration. Fura2 is one of the most frequently used ratiometric Ca2+ indicator.
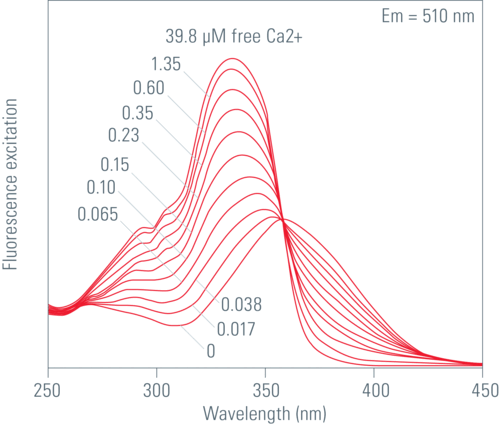
Fura2
The Ca2+ unbound form of Fura2 gets excited at 380 nm and the Ca2+ bound form at 340 nm. The emitted light is measured at around 510 nm. The fluorescence intensity increases at 340 nm with increasing Ca2+ concentration and decreases at 387 nm for the unbound form. There are some techniques to introduce the fluorescent Ca2+ indicator into cells, like ester loading, microinjection, chemical loading techniques or diffusion from patch-clamp pipettes.
For further information on different Ca2+ indicators, handling of the dye, labeling of the cells and cell loading, please refer to the Molecular Probes Handbook and Takahashi et al. (see References).
Benefit of ratiometric measurement
Artifacts due to uneven dye distribution, leakage of dye, photo bleaching and unequal cell thickness may be avoided by ratioing methods. In a typical experiment where you detect Ca2+ changes over time a calibration must be done. Calibration is possible in vitro or in vivo. For in vivo calibration the ratio of maximum and minimum Ca2+ concentrations is measured. These values may be obtained within the same experiment. For the maximum amount of bound Ca2+ indicator you may add an ionophore like Ionomycin or Bromo-A2318 to equilibrate the Ca2+ concentration. To get the minimum Ca2+ concentration you may apply an EGTA buffered solution to chelate all Ca2+.
Leica systems configuration
The system must be equipped with external filter wheels and EL6000 light source. The external filter wheels contain special excitation filters for Fura2 at 340 nm and 387 nm. Additionally you need the according filter cube consisting of a dichroic beamsplitter and an emission band pass filter for selection of the wavelength with the maximum at 510 nm.
Read more about how to set up a widefield calcium imaging experiment
Detailed information on:
- Setting of experimental conditions for imaging
- Define your time lapse experiment
- Fluorescence Intensity Ratio
- Calculation of the calcium concentration according to Grynkiewicz
you can find here:
Widefield Application Letter No. 01: Widefield Calcium Imaging with Calcium Indicator Fura2
Read more about ratiometric imaging
Ratiometric Imaging – Analyzing Ion Concentrations by Measuring Fluorophore Shifts
Ratiometric Imaging Needs a Specialized Setup
References
- Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260 (1985) 3440–3450.
- Takahashi A, Camacho P, Lechleiter JD, Herman B: Measurement of Intracellular Calcium. Physiological Rev 79 (1999) 1089.